Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test
- PMID: 32679298
- PMCID: PMC7336910
- DOI: 10.1016/j.jcv.2020.104539
Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test
Abstract
Background: Serological tests for anti-SARS-CoV-2 antibodies are becoming of great interest to determine seroprevalence in a given population, define previous exposure and identify highly reactive human donors for the generation of convalescent serum as therapeutic.
Objectives: We evaluated the diagnostic performance of the Abbott ARCHITECT SARS-CoV-2 IgG test, a fully automated indirect immunoassay that detects antibodies directed to a recombinant SARS-CoV-2 Nucleocapsid antigen.
Study design: Abbott ARCHITECT SARS-CoV-2 IgG immunoassay was compared to an indirect immunofluorescence assay (IFA) on sera from patients with COVID-19 collected at different days after symptoms onset or infected by other human coronaviruses. Comparison with neutralization test was also performed.
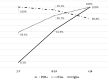
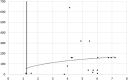
Results: After 7, 14 and >14 days after onset ARCHITECT was positive on 8.3 %; 61.9 % and 100 % of the tested samples compared to 58.3 %; 85.7 % and 100 % by IFA. The sensitivity was 72 % vs. IFA and 66.7 % vs. a real-time PCR, the specificity was 100 %. On 18 samples with neutralizing activity, 17 were positive by Abbott ARCHITECT SARS-CoV-2 IgG.
Conclusions: In our study, Abbott ARCHITECT SARS-CoV-2 IgG assay showed a satisfactory performance, with a very high specificity. IgG reactivity against SARSCoV-2 N antigen was detectable in all patients by two weeks after symptoms onset. In addition, concordance between this serological response and viral neutralization suggests that a strong humoral response may be predictive of a neutralization activity, regardless of the target antigens. This finding supports the use of this automated serological assay in diagnostic algorithm and public health intervention, especially for high loads of testing.
Keywords: CLIA; COVID-19; IgG; Immunofluorescence assay; SARS-CoV-2; Serological assay.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest Abbott Diagnostics supplied the materials employed for the purposes of this study. In no way this contribution influenced the study design and the analysis of the results. No additional conflict of interest or other competing relationships exist.
Figures
References
-
- World Health Organization . 2020. (WHO) Rolling Updates on Coronavirus Disease (COVID-19) Outbreak Situation.https://www.who.int/emergencies/diseases/novel-coronavirus-2019
-
- Centers for Disease Control and Prevention (CDC) Interim Guidelines for COVID-19 Antibody Testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-g... accessed June 20th, 2020.
-
- Gronvall G., Connell N., Kobokovich A. 2020. Developing a National Strategy for Serology (Antibody Testing) in the United States Johns Hopkins Center for Health Security; 621 E. Pratt Street, Suite 210, Baltimore, MD 21202; April 22nd.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

