Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature
- PMID: 32680541
- PMCID: PMC7368722
- DOI: 10.1186/s13256-020-02426-5
Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature
Abstract
Background: Premature ovarian failure is a relatively common condition that affects 1-3% of adult women. Premature ovarian failure occurs when there is loss of ovarian function in women younger than 40 years of age. The causes are mostly iatrogenic or idiopathic. Amenorrhea and infertility are the most important clinical manifestations. So far, no therapeutic intervention has been proved effective in restoring fertility in patients with premature ovarian failure. Attempts to stimulate ovarian function through hormone manipulation typically prove unsuccessful, and patients usually resort to egg donation to achieve pregnancy. In our preclinical work, intraovarian administration of human bone marrow-derived mesenchymal stem cells was able to restore ovarian hormone production, reactivate folliculogenesis, and reverse infertility in a chemotherapy-induced ovarian failure mouse model.
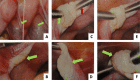
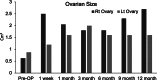
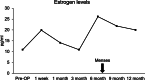
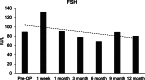
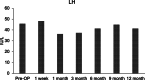
Case presentation: We present two cases of Caucasian women with premature ovarian failure who resumed ovarian estrogen production and menses 7 months following autologous bone marrow-derived mesenchymal stem cell injections into the ovary. This pilot clinical study is registered with ClinicalTrials.gov (identifier NCT02696889 ). In this report, we present data from our first two cases that have completed study procedures so far. The bone marrow-derived mesenchymal stem cells were harvested from the bone marrow of the iliac crest of the patients with premature ovarian failure and nucleated cells concentrated and enriched in bone marrow-derived mesenchymal stem cells intraoperatively, and then injected into the patient's right ovary via laparoscopy. Autologous bone marrow stem cell engraftment into the ovary resulted in several improvements in the treated patients with premature ovarian failure. In measurements by transvaginal ultrasound, there were increases of approximately 50% in volume of the treated ovaries in comparison with the contralateral control ovaries that persisted to the end of the study (1 year). Serum levels of estrogen increased by approximately 150% compared with the preoperative levels. Each of the two patients had an episode of menses, and also both of them reported marked improvement of their menopausal symptoms that also persisted to the end of the study (1 year). The bone marrow-derived mesenchymal stem cell implantation procedure was very well tolerated with no reported adverse events.
Conclusions: Our study reveals promising improvement of premature ovarian failure-related clinical manifestations in two patients after intraovarian autologous bone marrow-derived mesenchymal stem cells engraftment. These early observations call for additional assessment and further development of intraovarian bone marrow-derived mesenchymal stem cell injection for possible treatment of patients with premature ovarian failure.
Keywords: BMSCs; Bone marrow–derived stem cells; Case report; Cell therapy; Infertility; MSCs; Mesenchymal stem cells; Premature ovarian failure.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

