Early Experience of COVID-19 in a US Children's Hospital
- PMID: 32680880
- PMCID: PMC7546093
- DOI: 10.1542/peds.2020-003186
Early Experience of COVID-19 in a US Children's Hospital
Abstract
Objectives: We aim to describe the demographics, clinical presentation, hospital course, and severity of pediatric inpatients with coronavirus disease 2019 (COVID-19), with an emphasis on healthy, immunocompromised, and chronically ill children.
Methods: We conducted a single-center retrospective cohort study of hospitalized children aged younger than 22 years with COVID-19 infection at Steven and Alexandra Cohen Children's Medical Center at Northwell Health. Cases were identified from patients with fever and/or respiratory symptoms who underwent a nucleic acid amplification-based test for severe acute respiratory syndrome coronavirus 2.
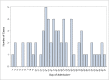
Results: Sixty-five patients were identified. The median age was 10.3 years (interquartile range, 1.4 months to 16.3 years), with 48% of patients older than 12 years and 29% of patients younger than 60 days of age. Fever was present in 86% of patients, lower respiratory symptoms or signs in 60%, and gastrointestinal symptoms in 62%. Thirty-five percent of patients required ICU care. The white blood cell count was elevated in severe disease (P = .0027), as was the C-reactive protein level (P = .0192), compared with mild and moderate disease. Respiratory support was required in 34% of patients. Severity was lowest in infants younger than 60 days of age and highest in chronically ill children; 79% of immunocompromised children had mild disease. One death was reported.
Conclusions: Among children who are hospitalized for COVID-19, most are younger than 60 days or older than 12 years of age. Children may have severe infection requiring intensive care support. The clinical course of immunocompromised patients was not more severe than that of other children. Elevated white blood cell count and C-reactive protein level are associated with greater illness severity.
Copyright © 2020 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Figures


References
-
- Dong Y, Mo X, Hu Y, et al. . Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

