Mechanisms of human embryo development: from cell fate to tissue shape and back
- PMID: 32680920
- PMCID: PMC7375473
- DOI: 10.1242/dev.190629
Mechanisms of human embryo development: from cell fate to tissue shape and back
Abstract
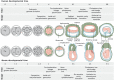
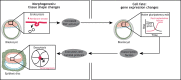
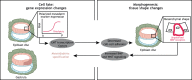
Gene regulatory networks and tissue morphogenetic events drive the emergence of shape and function: the pillars of embryo development. Although model systems offer a window into the molecular biology of cell fate and tissue shape, mechanistic studies of our own development have so far been technically and ethically challenging. However, recent technical developments provide the tools to describe, manipulate and mimic human embryos in a dish, thus opening a new avenue to exploring human development. Here, I discuss the evidence that supports a role for the crosstalk between cell fate and tissue shape during early human embryogenesis. This is a critical developmental period, when the body plan is laid out and many pregnancies fail. Dissecting the basic mechanisms that coordinate cell fate and tissue shape will generate an integrated understanding of early embryogenesis and new strategies for therapeutic intervention in early pregnancy loss.
Keywords: Aneuploidy; Cell fate; Embryonic stem cells; Human embryo; Morphogenesis; Polarisation.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

