Isoform- and Cell Type-Specific Roles of Glycogen Synthase Kinase 3 N-Terminal Serine Phosphorylation in Liver Ischemia Reperfusion Injury
- PMID: 32680958
- PMCID: PMC8788336
- DOI: 10.4049/jimmunol.2000397
Isoform- and Cell Type-Specific Roles of Glycogen Synthase Kinase 3 N-Terminal Serine Phosphorylation in Liver Ischemia Reperfusion Injury
Abstract
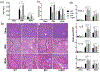
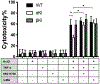
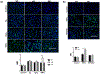
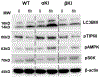
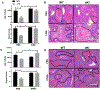
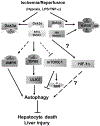
Glycogen synthase kinase 3 (Gsk3) α and β are both constitutively active and inhibited upon stimulation by N-terminal serine phosphorylation. Although roles of active Gsk3 in liver ischemia reperfusion injury (IRI) have been well appreciated, whether Gsk3 N-terminal serine phosphorylation has any functional significance in the disease process remains unclear. In a murine liver partial warm ischemia model, we studied Gsk3 N-terminal serine mutant knock-in (KI) mice and showed that liver IRI was decreased in Gsk3αS21A but increased in Gsk3βS9A mutant KI mice. Bone marrow chimeric experiments revealed that the Gsk3α, but not β, mutation in liver parenchyma protected from IRI, and both mutations in bone marrow-derived cells exacerbated liver injuries. Mechanistically, mutant Gsk3α protected hepatocytes from inflammatory (TNF-α) cell death by the activation of HIV-1 TAT-interactive protein 60 (TIP60)-mediated autophagy pathway. The pharmacological inhibition of TIP60 or autophagy diminished the protection of the Gsk3α mutant hepatocytes from inflammatory cell death in vitro and the Gsk3α mutant KI mice from liver IRI in vivo. Thus, Gsk3 N-terminal serine phosphorylation inhibits liver innate immune activation but suppresses hepatocyte autophagy in response to inflammation. Gsk3 αS21, but not βS9, mutation is sufficient to sustain Gsk4 activities in hepatocytes and protect livers from IRI via TIP60 activation.
Copyright © 2020 by The American Association of Immunologists, Inc.
Figures




References
-
- Kaczorowski DJ, Tsung A, and Billiar TR. 2009. Innate immune mechanisms in ischemia/reperfusion. Front Biosci (Elite Ed) 1: 91–98. - PubMed
-
- Zhai Y, Busuttil RW, and Kupiec-Weglinski JW. 2011. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 11: 1563–1569. - PMC - PubMed
-
- Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, and Billiar TR. 2005. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol 175: 7661–7668. - PubMed
-
- Zhai Y, Shen X. d., O’Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, and Kupiec-Weglinski JW. 2004. Cutting Edge: TLR4 Activation Mediates Liver Ischemia/Reperfusion Inflammatory Response via IFN Regulatory Factor 3-Dependent MyD88-Independent Pathway. The Journal of Immunology 173: 7115–7119. - PubMed
-
- Wu HS, Zhang JX, Wang L, Tian Y, Wang H, and Rotstein O. 2004. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int 3: 250–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

