A clinical study of patients with novel CDHR1 genotypes associated with late-onset macular dystrophy
- PMID: 32681094
- PMCID: PMC8182786
- DOI: 10.1038/s41433-020-1045-3
A clinical study of patients with novel CDHR1 genotypes associated with late-onset macular dystrophy
Abstract
Purpose: To describe the clinical and electrophysiological features of adult-onset macular dystrophy, due to novel combinations of CDHR1 alleles, and compare the associated phenotypes with previous reports.
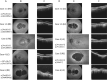
Methods: The clinical records of patients with macular dystrophy and biallelic variants in CDHR1 were reviewed. Data analysed included best corrected visual acuity (BCVA), fundus images: autofluorescence (AF) and optical coherence tomography (OCT); full field electroretinography (ERG) and pattern ERG (PERG).
Results: Seven patients from six pedigrees were ascertained. One patient was homozygous for a known synonymous variant p.(Pro261=), four were compound heterozygous for the p.(Pro261=) variant and a novel allele of CDHR1: p.(Gly188Ser), p.(Met1?), or p.(Val458Asp); one patient was compound heterozygous for two previously unreported variants: c.297+1G>T in trans with p.(Pro735Thr). The range of BCVA at the last clinic review was (6/5-6/60). Autofluorescence showed macular flecks of increased AF in mild cases and patches of reduced AF in severe cases. The OCT showed attenuation of the ellipsoid zone (EZ) in mild cases and loss of the EZ and the outer nuclear layer in severe cases; one patient had subfoveal hyporeflective region between the EZ and the retinal pigment epithelium. The full field ERG was normal or borderline subnormal in all cases, and the PERG was subnormal in mild cases or undetectable in severe cases.
Conclusions: This report corroborates previous observations that genotypes distinct from those causing pan-retinal dystrophy can cause a milder phenotype, predominantly affecting the macula, and expands the spectrum of these genotypes. The findings in this cohort suggest a potential macular susceptibility to mild perturbations of the photoreceptor cadherin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Comment in
-
CDHR1-related late-onset macular dystrophy: further insights.Eye (Lond). 2021 Oct;35(10):2901-2902. doi: 10.1038/s41433-020-01212-3. Epub 2020 Oct 1. Eye (Lond). 2021. PMID: 33005045 Free PMC article. No abstract available.
References
-
- Glöckle N, Kohl S, Mohr J, Scheurenbrand T, Sprecher A, Weisschuh N, et al. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. Eur J Hum Genet. 2014;22:99–104. doi: 10.1038/ejhg.2013.72. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

