Delivering the power of nanomedicine to patients today
- PMID: 32681950
- PMCID: PMC7362824
- DOI: 10.1016/j.jconrel.2020.07.007
Delivering the power of nanomedicine to patients today
Abstract
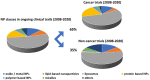
The situation of the COVID-19 pandemic reminds us that we permanently need high-value flexible solutions to urgent clinical needs including simplified diagnostic technologies suitable for use in the field and for delivering targeted therapeutics. From our perspective nanotechnology is revealed as a vital resource for this, as a generic platform of technical solutions to tackle complex medical challenges. It is towards this perspective and focusing on nanomedicine that we take issue with Prof Park's recent editorial published in the Journal of Controlled Release. Prof. Park argued that in the last 15 years nanomedicine failed to deliver the promised innovative clinical solutions to the patients (Park, K. The beginning of the end of the nanomedicine hype. Journal of Controlled Release, 2019; 305, 221-222 [1]. We, the ETPN (European Technology Platform on Nanomedicine) [2], respectfully disagree. In fact, the more than 50 formulations currently in the market, and the recent approval of 3 key nanomedicine products (e. g. Onpattro, Hensify and Vyxeos), have demonstrated that the nanomedicine field is concretely able to design products that overcome critical barriers in conventional medicine in a unique manner, but also to deliver within the cells new drug-free therapeutic effects by using pure physical modes of action, and therefore make a difference in patients lives. Furthermore, the >400 nanomedicine formulations currently in clinical trials are expecting to bring novel clinical solutions (e.g. platforms for nucleic acid delivery), alone or in combination with other key enabling technologies to the market, including biotechnologies, microfluidics, advanced materials, biomaterials, smart systems, photonics, robotics, textiles, Big Data and ICT (information & communication technologies) more generally. However, we agree with Prof. Park that " it is time to examine the sources of difficulty in clinical translation of nanomedicine and move forward ". But for reaching this goal, the investments to support clinical translation of promising nanomedicine formulations should increase, not decrease. As recently encouraged by EMA in its roadmap to 2025, we should create more unity through a common knowledge hub linking academia, industry, healthcare providers and hopefully policy makers to reduce the current fragmentation of the standardization and regulatory body landscape. We should also promote a strategy of cross-technology innovation, support nanomedicine development as a high value and low-cost solution to answer unmet medical needs and help the most promising innovative projects of the field to get better and faster to the clinic. This global vision is the one that the ETPN chose to encourage for the last fifteen years. All actions should be taken with a clear clinical view in mind, " without any fanfare", to focus "on what matters in real life", which is the patient and his/her quality of life. This ETPN overview of achievements in nanomedicine serves to reinforce our drive towards further expanding and growing the maturity of nanomedicine for global healthcare, accelerating the pace of transformation of its great potential into tangible medical breakthroughs.
Keywords: Clinical translation; Healthtech; Nanomedicine; Nanotechnology; Regulatory; Standardization.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Matthieu GERMAIN and Agnes POTTIER are co-inventors of patent applications related to Hensify product (Nanobiotix) described in this article. Alexandre CECCALDI is employee of ETPN. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. Credit authors statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

