Amphetamine maintenance therapy during intermittent cocaine self-administration in rats attenuates psychomotor and dopamine sensitization and reduces addiction-like behavior
- PMID: 32682325
- PMCID: PMC7853073
- DOI: 10.1038/s41386-020-0773-1
Amphetamine maintenance therapy during intermittent cocaine self-administration in rats attenuates psychomotor and dopamine sensitization and reduces addiction-like behavior
Abstract
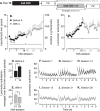
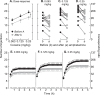
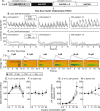
D-amphetamine maintenance therapy shows promise as a treatment for people with cocaine addiction. Preclinical studies using Long Access (LgA) cocaine self-administration procedures suggest D-amphetamine may act by preventing tolerance to cocaine's effects at the dopamine transporter (DAT). However, Intermittent Access (IntA) cocaine self-administration better reflects human patterns of use, is especially effective in promoting addiction-relevant behaviors, and instead of tolerance, produces psychomotor, incentive, and neural sensitization. We asked, therefore, how D-amphetamine maintenance during IntA influences cocaine use and cocaine's potency at the DAT. Male rats self-administered cocaine intermittently (5 min ON, 25 min OFF x10; 5-h/session) for 14 sessions, with or without concomitant D-amphetamine maintenance therapy during these 14 sessions (5 mg/kg/day via s.c. osmotic minipump). We then assessed responding for cocaine under a progressive ratio schedule, responding under extinction and cocaine-primed reinstatement of drug seeking. We also assessed the ability of cocaine to inhibit dopamine uptake in the nucleus accumbens core using fast scan cyclic voltammetry ex vivo. IntA cocaine self-administration produced psychomotor (locomotor) sensitization, strong motivation to take and seek cocaine, and it increased cocaine's potency at the DAT. D-amphetamine co-administration suppressed the psychomotor sensitization produced by IntA cocaine experience. After cessation of D-amphetamine treatment, the motivation to take and seek cocaine was also reduced, and sensitization of cocaine's actions at the DAT was reversed. Thus, treatment with D-amphetamine might reduce cocaine use by preventing sensitization-related changes in cocaine potency at the DAT, consistent with an incentive-sensitization view of addiction.
Figures


Comment in
-
D-amphetamine maintenance treatment goes a long way: lasting therapeutic effects on cocaine behavioral effects and cocaine potency at the dopamine transporter.Neuropsychopharmacology. 2021 Jan;46(2):275-276. doi: 10.1038/s41386-020-00825-2. Epub 2020 Aug 28. Neuropsychopharmacology. 2021. PMID: 32859997 Free PMC article. No abstract available.
References
-
- Fischer B, Blanken P, Da Silveira D, Gallassi A, Goldner EM, Rehm J, et al. Effectiveness of secondary prevention and treatment interventions for crack-cocaine abuse: a comprehensive narrative overview of English-language studies. Int J Drug Policy. 2015;26:352–63. doi: 10.1016/j.drugpo.2015.01.002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

