The Paradox of HIV Blood-Brain Barrier Penetrance and Antiretroviral Drug Delivery Deficiencies
- PMID: 32682564
- PMCID: PMC7483662
- DOI: 10.1016/j.tins.2020.06.007
The Paradox of HIV Blood-Brain Barrier Penetrance and Antiretroviral Drug Delivery Deficiencies
Abstract
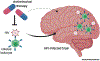
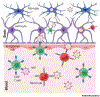
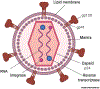
HIV attacks the body's immune cells, frequently compromises the integrity of the blood-brain barrier (BBB), and infects the CNS in the early stages of infection. Dysfunction of the BBB further potentiates viral replication within the CNS, which can lead to HIV-associated neuropathology. Antiretroviral therapy (ART) significantly improves HIV patient outcomes and reduces mortality rates. However, there has been limited progress in targeting latent viral reservoirs within the CNS, which may eventually lead to rebound viremia. While ART drugs are shown to be effective in attenuating HIV replication in the periphery, the protection of the brain by the BBB offers an isolated sanctuary to harbor HIV and maintains chronic and persistent replication within the CNS. In this review, we elucidate the pathology of the BBB, its ability to potentiate viral replication, as well as current therapies and insufficiencies in treating HIV-infected individuals.
Keywords: HIV; antiretroviral therapy; blood–brain barrier; drug delivery; nanomedicine.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Centers for Disease Control and Prevention (2018) People with diagnosed HIV In Diagnoses of HIV Infection in the United States and Dependent Areas, 2018, CDC
-
- Joseph J et al. (2016) HIV-1 induced CNS dysfunction: current overview and research priorities. Curr. HIV Res 14, 389–399 - PubMed
-
- Abbott NJ et al. (2010) Structure and function of the blood-brain barrier. Neurobiol. Dis 37, 13–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

