Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18)
- PMID: 32683565
- PMCID: PMC7383036
- DOI: 10.1007/s10549-020-05755-7
Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18)
Abstract
Purpose: Palbociclib is a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, approved in combination with endocrine therapy for the treatment of women and men with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer (HR+/HER2- ABC). In the phase 2, open-label, PALOMA-1 trial, palbociclib plus letrozole significantly prolonged progression-free survival (PFS) versus letrozole alone (hazard ratio, 0.488; 95% CI 0.319‒0.748; P = 0.0004; median PFS, 20.2 vs 10.2 months, respectively) in postmenopausal women with estrogen receptor-positive (ER+)/HER2- ABC. Here, we present the final overall survival (OS) and updated safety results.
Methods: Postmenopausal women with ER+/HER2- ABC were randomized 1:1 to receive either palbociclib (125 mg/day, 3/1 schedule) plus letrozole (2.5 mg/day, continuous) or letrozole alone (2.5 mg/day, continuous). The primary endpoint was investigator-assessed PFS; secondary endpoints included OS and safety.
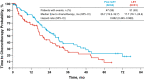
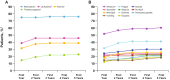
Results: A total of 165 patients were randomized. At the data cutoff date of December 30, 2016 (median duration of follow-up, 64.7 months), the stratified hazard ratio for OS was 0.897 (95% CI 0.623-1.294; P = 0.281); median OS in the palbociclib plus letrozole and letrozole alone arms was 37.5 and 34.5 months, respectively. The median time from randomization to first subsequent chemotherapy use was longer with palbociclib plus letrozole than letrozole alone (26.7 and 17.7 months, respectively). The most frequently reported adverse event in the palbociclib plus letrozole arm was neutropenia (any grade, 75%; grade 3 or 4, 59%).
Conclusions: Palbociclib plus letrozole treatment led to a numerical but not statistically significant improvement in median OS. Pfizer Inc (NCT00721409).
Keywords: Advanced breast cancer; ER+/HER2−; Letrozole; Overall survival; Palbociclib.
Conflict of interest statement
R.S. Finn has received consulting fees from Pfizer Inc, AstraZeneca, Bayer, Novartis, Bristol-Myers Squibb, Eisai, Eli Lilly, Merck, and Roche, as well as other research funding from Pfizer Inc and honoraria from Bayer, Pfizer Inc, Bristol-Myers Squibb, Novartis, Eisai, and Eli Lilly. K. Boer has received consulting fees from Pfizer Inc, Novartis, Eli Lilly, and Roche and served on speakers’ bureaus for Pfizer Inc, Novartis, Eli Lilly, AstraZeneca, and Roche. I. Bondarenko, Y. Shparyk, A. Thummala, and N. Voitko have nothing to report. R. Patel is a stockholder of Novartis. J. Ettl received consulting fees from Eli Lilly, Novartis, Pfizer Inc, Roche, and Eisai; performed contracted research for Celgene; and received honoraria and travel support from Celgene, Eli Lilly, Novartis, Pfizer Inc, Roche, and Teva. T. Pinter has received consulting fees from Amgen and Pfizer Inc and served on speakers’ bureaus for Roche, Bayer, Amgen, and Pfizer Inc. M. Schmidt has received honoraria and consulting fees from AstraZeneca, Celgene, Eisai, Eli Lilly, Myelo Therapeutics, Novartis, Pantarhei Oncology, Roche, Pfizer Inc, and Pierre Fabre; has received travel reimbursement from BioNTech, Pfizer Inc, and Roche; and has patents pending with Sividon. D.J. Slamon reports consultant/advisory roles for Pfizer Inc, Eli Lilly, and Novartis; stock ownership in Pfizer Inc; and research funding from Pfizer Inc and Novartis. E. Bananis, L. McRoy, K. Wilner, X. Huang, and S. Kim are employees and stockholders of Pfizer Inc.
Figures

References
-
- American Cancer Society (2017) Breast cancer facts & figures 2017–2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-.... Accessed 27 Aug 2019
-
- Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN, Jahanzeb M, Johnston SR, Korde LA, Khatcheressian JL, Mehta RS, Muss HB, Burstein HJ. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. - DOI - PubMed
-
- National Comprehensive Cancer Network (2019) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer Version 1.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 21 Feb 2020
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

