Knockout of sulfatase 2 is associated with decreased steatohepatitis and fibrosis in a mouse model of nonalcoholic fatty liver disease
- PMID: 32683952
- PMCID: PMC7509257
- DOI: 10.1152/ajpgi.00150.2019
Knockout of sulfatase 2 is associated with decreased steatohepatitis and fibrosis in a mouse model of nonalcoholic fatty liver disease
Abstract
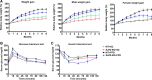
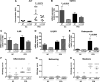
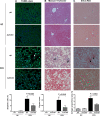
Sulfatase 2 (SULF2) is a heparan sulfate editing enzyme that regulates the milieu of growth factors and cytokines involved in a variety of cellular processes. We used a murine model of diet-induced steatohepatitis to assess the effect of SULF2 downregulation on the development of nonalcoholic steatohepatitis (NASH) and liver fibrosis. Wild-type B6;129 mice (WT) and Sulf2-knockout B6;129P2-SULF2Gt(PST111)Byg mice (Sulf2-KO) were fed a fast-food diet (FFD) rich in saturated fats, cholesterol, and fructose or a standard chow diet (SC) ad libitum for 9 mo. WT mice on FFD showed a threefold increase in hepatic Sulf2 mRNA expression, and a 2.2-fold increase in hepatic SULF2 protein expression compared with WT mice on SC. Knockout of Sulf2 led to a significant decrease in diet-mediated weight gain and dyslipidemia compared with WT mice on FFD. Knockout of Sulf2 also abrogated diet-induced steatohepatitis and hepatic fibrosis compared with WT mice on FFD. Furthermore, expression levels of the profibrogenic receptors TGFβR2 and PDGFRβ were significantly decreased in Sulf2-KO mice compared with WT mice on FFD. Together, our data suggest that knockout of Sulf2 significantly downregulates dyslipidemia, steatohepatitis, and hepatic fibrosis in a diet-induced mouse model of NAFLD, suggesting that targeting of SULF2 signaling may be a potential therapeutic mechanism in NASH.NEW & NOTEWORTHY We report for the first time that in wild-type (WT) mice, fast-food diet (FFD) induced a threefold increase in hepatic Sulf2 mRNA and a 2.2-fold increase in sulfatase 2 (SULF2) protein expression compared with WT mice on standard chow diet (SC). We showed that knockout of SULF2 ameliorates FFD-induced obesity, hyperlipidemia, steatohepatitis, and fibrosis. These data, along with work from other laboratories, suggest that SULF2 may be critical to the ability of the liver to progress to nonalcoholic steatohepatitis and fibrosis in conditions of overnutrition.
Keywords: SULF2; fast-food diet; liver fibrosis; nonalcoholic fatty liver disease; nonalcoholic steatohepatitis; obesity; sulfatase.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Asgharpour A, Cazanave SC, Pacana T, Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min HK, Mirshahi F, Bedossa P, Sun X, Hoshida Y, Koduru SV, Contaifer D Jr, Warncke UO, Wijesinghe DS, Sanyal AJ. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J Hepatol 65: 579–588, 2016. doi:10.1016/j.jhep.2016.05.005. - DOI - PMC - PubMed
-
- Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fastifood diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol 301: G825–G834, 2011. doi:10.1152/ajpgi.00145.2011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR001863/TR/NCATS NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- DK084567/Mayo Clinic Center for Cell Signaling and GI/International
- P30 DK084567/DK/NIDDK NIH HHS/United States
- CA210964/HHS | National Institutes of Health (NIH)/International
- P50 CA210964/CA/NCI NIH HHS/United States
- N01 CA015083/CA/NCI NIH HHS/United States
- CA100882/HHS | National Institutes of Health (NIH)/International
- CA15083/Mayo Clinic Cancer Center/International
- CA165076/HHS | National Institutes of Health (NIH)/International
- CA128633/HHS | National Institutes of Health (NIH)/International
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

