A listeriolysin O subunit vaccine is protective against Listeria monocytogenes
- PMID: 32684498
- PMCID: PMC7788512
- DOI: 10.1016/j.vaccine.2020.06.049
A listeriolysin O subunit vaccine is protective against Listeria monocytogenes
Abstract
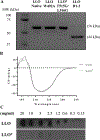
Listeria monocytogenes is a facultative intracellular pathogen responsible for the life-threatening disease listeriosis. The pore-forming toxin listeriolysin O (LLO) is a critical virulence factor that plays a major role in the L. monocytogenes intracellular lifecycle and is indispensable for pathogenesis. LLO is also a dominant antigen for T cells involved in sterilizing immunity and it was proposed that LLO acts as a T cell adjuvant. In this work, we generated a novel full-length LLO toxoid (LLOT) in which the cholesterol-recognition motif, a threonine-leucine pair located at the tip of the LLO C-terminal domain, was substituted with two glycine residues. We showed that LLOT lost its ability to bind cholesterol and to form pores. Importantly, LLOT retained binding to the surface of epithelial cells and macrophages, suggesting that it could efficiently be captured by antigen-presenting cells. We then determined if LLOT can be used as an antigen and adjuvant to protect mice from L. monocytogenes infection. Mice were immunized with LLOT alone or together with cholera toxin or Alum as adjuvants. We found that mice immunized with LLOT alone or in combination with the Th2-inducing adjuvant Alum were not protected against L. monocytogenes. On the other hand, mice immunized with LLOT along with the experimental adjuvant cholera toxin, were protected against L. monocytogenes, as evidenced by a significant decrease in bacterial burden in the liver and spleen three days post-infection. This immunization regimen elicited mixed Th1, Th2, and Th17 responses, as well as the generation of LLO-neutralizing antibodies. Further, we identified T cells as being required for immunization-induced reductions in bacterial burden, whereas B cells were dispensable in our model of non-pregnant young mice. Overall, this work establishes that LLOT is a promising vaccine antigen for the induction of protective immunity against L. monocytogenes by subunit vaccines containing Th1-driving adjuvants.
Keywords: Cancer immunotherapy; Cholesterol-dependent cytolysin; Listeria monocytogenes; Listeriolyin O; Vaccine.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




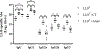
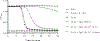
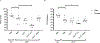
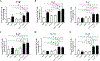
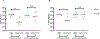
References
-
- Vazquez-Boland JA, Dominguez-Bernal G, Gonzalez-Zorn B, Kreft J & Goebel W Pathogenicity islands and virulence evolution in Listeria. Microbes Infect 3, 571–584 (2001). - PubMed
-
- Teberg AJ, Yonekura ML, Salminen C & Pavlova Z Clinical manifestations of epidemic neonatal listeriosis. Pediatr Infect Dis J 6, 817–820 (1987). - PubMed
-
- Mylonakis E, Paliou M, Hohmann EL, Calderwood SB & Wing EJ Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 81, 260–269 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

