Nuclear MYH9-induced CTNNB1 transcription, targeted by staurosporin, promotes gastric cancer cell anoikis resistance and metastasis
- PMID: 32685004
- PMCID: PMC7359096
- DOI: 10.7150/thno.46001
Nuclear MYH9-induced CTNNB1 transcription, targeted by staurosporin, promotes gastric cancer cell anoikis resistance and metastasis
Abstract
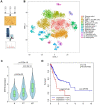
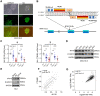
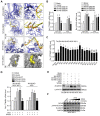
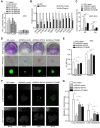
Rationale: Peritoneal metastasis predicts poor prognosis of gastric cancer (GC) patients, and the underlying mechanisms are poorly understood. Methods: The 2-DIGE, MALDI-TOF/TOF MS and single-cell transcriptome were used to detect differentially expressed proteins among normal gastric mucosa, primary GC and peritoneal metastatic tissues. Lentiviruses carrying shRNA and transcription activator-like effector nuclease technology were used to knock down myosin heavy chain 9 (MYH9) expression in GC cell lines. Immunofluorescence, immune transmission electron microscopy, chromatin fractionation, co-immunoprecipitation, and assays for chromatin immunoprecipitation, dual luciferase reporter, agarose-oligonucleotide pull-down, flow cytometry and cell anoikis were performed to uncover nuclear MYH9-induced β-catenin (CTNNB1) transcription in vitro. Nude mice and conditional transgenic mice were used to investigate the findings in vivo. Results: We observed that MYH9 was upregulated in metastatic GC tissues and was associated with a poor prognosis of GC patients. Mechanistically, we confirmed that MYH9 was mainly localized in the GC cell nuclei by four potential nuclear localization signals. Nuclear MYH9 bound to the CTNNB1 promoter through its DNA-binding domain, and interacted with myosin light chain 9, β-actin and RNA polymerase II to promote CTNNB1 transcription, which conferred resistance to anoikis in GC cells in vitro and in vivo. Staurosporine reduced nuclear MYH9 S1943 phosphorylation to inhibit CTNNB1 transcription, Wnt/β-catenin signaling activation and GC progression in both orthotropic xenograft GC nude mouse and transgenic GC mouse models. Conclusion: This study identified that nuclear MYH9-induced CTNNB1 expression promotes GC metastasis, which could be inhibited by staurosporine, indicating a novel therapy for GC peritoneal metastasis.
Keywords: CTNNB1; MYH9; anoikis resistance; gastric cancer; metastasis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Hu Y, Huang C, Sun Y, Su X, Cao H, Hu J. et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol. 2016;34:1350–7. - PubMed
-
- Alyami M, Hubner M, Grass F, Bakrin N, Villeneuve L, Laplace N. et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20:E368–E77. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33:1760–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

