Exosomes-mediated Transfer of miR-125a/b in Cell-to-cell Communication: A Novel Mechanism of Genetic Exchange in the Intestinal Microenvironment
- PMID: 32685005
- PMCID: PMC7359098
- DOI: 10.7150/thno.41802
Exosomes-mediated Transfer of miR-125a/b in Cell-to-cell Communication: A Novel Mechanism of Genetic Exchange in the Intestinal Microenvironment
Abstract
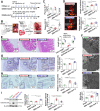
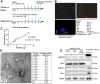
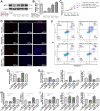
Glucagon-like peptide-2 (GLP-2), a key factor in intestinal rehabilitation therapy of short bowel syndrome (SBS), may require cell-to-cell communication to exert its biological functions. However, understanding of the mechanism remains elusive. Here, we report participation of exosomal miR-125a/b in GLP-2 mediated intestinal epithelial cells-myofibroblasts cross-talk in intestinal microenvironment. Methods: The effects of GLP-2 on the proliferation and apoptosis of intestinal epithelial cells in SBS rat models were evaluated. Exosomes were extracted from residual jejunum tissue of GLP-2 or vehicle treated SBS rats using ultracentrifugation method, and identified by nanoparticle trafficking analysis (NTA), transmission electron microscopy and western blotting. miRNA sequencing combined with qRT-PCR validation were used to identify differentially expressed miRNAs. miRNAs, which might be involved in proliferation and apoptosis of intestinal epithelial cells, were screened and further verified by miRNA functional experiments. Moreover, the proliferation-promoting and anti-apoptosis effects of GLP-2 on intestinal myofibroblasts, which expressing GLP-2 receptor, and whether GLP-2 could influence the content of miRNAs in the derived exosomes were studied. The downstream pathways were explored by miRNA function recovery experiment, luciferase reporter assay, pull down experiment, knockdown and overexpression of target gene and other experiments based on the bioinformatics prediction of miRNA target gene. Results: GLP-2 significantly promoted intestinal growth, facilitated the proliferation of intestinal crypt epithelial cells and inhibited the apoptosis of intestinal villi epithelial cells in type II SBS rats. GLP-2 significantly down-regulated exosomal miR-125a/b both in residual jejunums derived exosomes and in exosomes secreted by GLP-2R positive cells. Exosomal miR-125a/b was responsible for GLP-2 mediated intestinal epithelial cells proliferation promotion and apoptosis attenuation. miR-125a/b inhibited the proliferation and promotes apoptosis of intestinal epithelial cells by suppressing the myeloid cell leukemia-1 (MCL1). Conclusions: miR-125a/b shuttled by intestinal myofibroblasts derived exosomes regulate the proliferation and apoptosis of intestinal epithelial cells. GLP-2 treatment significantly decreases the level of miR-125a/b in the exosomes of intestinal myofibroblasts. miR-125a/b modulates the proliferation and apoptosis of intestinal epithelial cells by targeting the 3'UTR region of MCL1. Hence, this study indicates a novel mechanism of genetic exchange between cells in intestinal microenvironment.
Keywords: Exosomes; Glucagon-like peptide-2; Proliferation and apoptosis of intestinal epithelial cells; Short bowel syndrome; miR-125a/b.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







Similar articles
-
Upregulation of proapoptotic microRNA mir-125a after massive small bowel resection in rats.Ann Surg. 2012 Apr;255(4):747-53. doi: 10.1097/SLA.0b013e31824b485a. Ann Surg. 2012. PMID: 22418008 Free PMC article.
-
Exosome microRNA-125a-5p derived from epithelium promotes M1 macrophage polarization by targeting IL1RN in chronic obstructive pulmonary disease.Int Immunopharmacol. 2024 Aug 20;137:112466. doi: 10.1016/j.intimp.2024.112466. Epub 2024 Jun 13. Int Immunopharmacol. 2024. PMID: 38875998
-
Downregulation of MicroRNA-125a in Placenta Accreta Spectrum Disorders Contributes Antiapoptosis of Implantation Site Intermediate Trophoblasts by Targeting MCL1.Reprod Sci. 2019 Dec;26(12):1582-1589. doi: 10.1177/1933719119828040. Epub 2019 Feb 19. Reprod Sci. 2019. PMID: 30782086
-
Gut hormones, and short bowel syndrome: the enigmatic role of glucagon-like peptide-2 in the regulation of intestinal adaptation.World J Gastroenterol. 2006 Jul 14;12(26):4117-29. doi: 10.3748/wjg.v12.i26.4117. World J Gastroenterol. 2006. PMID: 16830359 Free PMC article. Review.
-
Enterohormone therapy for short bowel syndrome.Curr Opin Endocrinol Diabetes Obes. 2022 Apr 1;29(2):207-218. doi: 10.1097/MED.0000000000000710. Curr Opin Endocrinol Diabetes Obes. 2022. PMID: 35034035 Review.
Cited by
-
The effects of tumor-derived exosomes enriched with miRNA-211a on B16F10 cells.Contemp Oncol (Pozn). 2024;28(2):121-129. doi: 10.5114/wo.2024.142364. Epub 2024 Aug 23. Contemp Oncol (Pozn). 2024. PMID: 39421705 Free PMC article.
-
Exosome-mediated miR-7-5p delivery enhances the anticancer effect of Everolimus via blocking MNK/eIF4E axis in non-small cell lung cancer.Cell Death Dis. 2022 Feb 8;13(2):129. doi: 10.1038/s41419-022-04565-7. Cell Death Dis. 2022. PMID: 35136028 Free PMC article.
-
Exosome tropism and various pathways in lung cancer metastasis.Front Immunol. 2025 Feb 14;16:1517495. doi: 10.3389/fimmu.2025.1517495. eCollection 2025. Front Immunol. 2025. PMID: 40028322 Free PMC article. Review.
-
Tumor-derived exosomes encapsulating miR-34a promote apoptosis and inhibit migration and tumor progression of colorectal cancer cells under in vitro condition.Daru. 2021 Dec;29(2):267-278. doi: 10.1007/s40199-021-00400-0. Epub 2021 Aug 17. Daru. 2021. PMID: 34405380 Free PMC article.
-
Purple sweet potato delphinidin-3-rutin represses glioma proliferation by inducing miR-20b-5p/Atg7-dependent cytostatic autophagy.Mol Ther Oncolytics. 2022 Jul 31;26:314-329. doi: 10.1016/j.omto.2022.07.007. eCollection 2022 Sep 15. Mol Ther Oncolytics. 2022. PMID: 36090477 Free PMC article.
References
-
- Mouillot T, Boehm V, Treton X, Ferrandi E, Kapel N, Cazals-Hatem D. et al. Small-Bowel Adaptation: A Case of Morphological Changes Induced by Teduglutide in Short-Bowel Syndrome With Intestinal Failure. JPEN J Parenter Enteral Nutr. 2020 [Epub ahead of print] - PubMed
-
- Pironi L, Arends J, Bozzetti F, Cuerda C, Gillanders L, Jeppesen PB. et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35:247–307. - PubMed
-
- Billiauws L, Joly F. Emerging treatments for short bowel syndrome in adult patients. Expert Rev Gastroenterol Hepatol. 2019;13:241–6. - PubMed
-
- Norsa L, Artru S, Lambe C, Talbotec C, Pigneur B, Ruemmele F. et al. Long term outcomes of intestinal rehabilitation in children with neonatal very short bowel syndrome: Parenteral nutrition or intestinal transplantation. Clin Nutr. 2019;38:926–33. - PubMed
-
- Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol. 1997;272:E1050–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

