Tandem CAR-T cells targeting CD70 and B7-H3 exhibit potent preclinical activity against multiple solid tumors
- PMID: 32685008
- PMCID: PMC7359081
- DOI: 10.7150/thno.43991
Tandem CAR-T cells targeting CD70 and B7-H3 exhibit potent preclinical activity against multiple solid tumors
Abstract
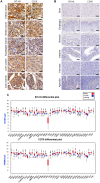
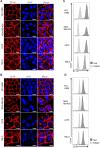
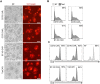
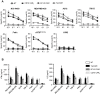
Purpose: Given that heterogeneous expression and variants of antigens on solid tumors are responsible for relapse after chimeric antigen receptor (CAR)-T cell therapy, we hypothesized that combinatorial targeting two tumor-associated antigens would lessen this problem and enhance the antitumor activity of T cells. Methods: The co-expression level of CD70 and B7-H3 was analyzed in multiple tumor tissue samples. Further, two putative antigens were identified in The Cancer Genome Atlas and Gene Expression Profiling Interactive Analysis database. Two CD70 targeted CARs with different antigen binding domain, truncated CD27 and CD70 specific single-chain antibody fragment (scFv), were designed to screen a more suitable target-antigen binding moiety. Accordingly, we designed a bivalent tandem CAR (TanCAR) and further assessed the anti-tumor efficacy of TanCAR-T cells in vitro and in vivo. Results: Our results indicated that co-expression of CD70 and B7-H3 was observed on multiple tumor types including kidney, breast, esophageal, liver, colon cancer, glioma as well as melanoma. The CD70 targeted CAR-T cells with binding moiety of CD70 specific scFv exhibit a higher affinity and antitumor effect against CD70+ tumor cells. TanCAR-T cells induced enhanced ability of cytolysis and cytokine release over unispecific CAR-T cells when encountering tumor cells expressing two target-antigens. Further, low doses of TanCAR-T cells could also effectively control the lung cancer and melanoma xenografts and improved overall survival of the treated animals. Conclusion: TanCAR-T cells targeting CD70 and B7-H3 exhibit enhanced antitumor functionality and improve the problem of antigenic heterogeneity and variant in the treatment against solid tumor and melanoma.
Keywords: B7-H3; CD70; Chimeric antigen receptor T cell; Immunotherapy; Solid tumor.
© The author(s).
Conflict of interest statement
Competing Interests: Aiping Tong, Gang Guo and Liangxue Zhou have filed patents related to this work. Other authors declare no competing interests.
Figures


References
-
- Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2018;15:31–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

