Mitogen- and Stress-Activated Protein Kinase 1 Mediates Alcohol-Upregulated Transcription of Brf1 and tRNA Genes to Cause Phenotypic Alteration
- PMID: 32685086
- PMCID: PMC7336232
- DOI: 10.1155/2020/2067959
Mitogen- and Stress-Activated Protein Kinase 1 Mediates Alcohol-Upregulated Transcription of Brf1 and tRNA Genes to Cause Phenotypic Alteration
Abstract
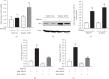
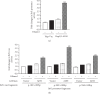
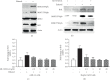
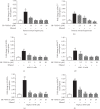
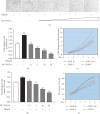
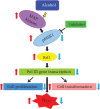
Upregulation of Brf1 (TFIIB-related factor 1) and Pol III gene (RNA polymerase III-dependent gene, such as tRNAs and 5S rRNA) activities is associated with cell transformation and tumor development. Alcohol intake causes liver injury, such as steatosis, inflammation, fibrosis, and cirrhosis, which enhances the risk of HCC development. However, the mechanism of alcohol-promoted HCC remains to be explored. We have designed the complementary research system, which is composed of cell lines, an animal model, human samples, and experiments in vivo and in vitro, to carry out this project by using molecular biological, biochemical, and cellular biological approaches. It is a unique system to explore the mechanism of alcohol-associated HCC. Our results indicate that alcohol upregulates Brf1 and Pol III gene (tRNAs and 5S rRNA) transcription in primary mouse hepatocytes, immortalized mouse hepatocyte-AML-12 cells, and engineered human HepG2-ADH cells. Alcohol activates MSK1 to upregulate expression of Brf1 and Pol III genes, while inhibiting MSK1 reduces transcription of Brf1 and Pol III genes in alcohol-treated cells. The inhibitor of MSK1, SB-747651A, decreases the rates of cell proliferation and colony formation. Alcohol feeding promotes liver tumor development of the mouse. These results, for the first time, show the identification of the alcohol-response promoter fragment of the Pol III gene key transcription factor, Brf1. Our studies demonstrate that Brf1 expression is elevated in HCC tumor tissues of mice and humans. Alcohol increases cellular levels of Brf1, resulting in enhancement of Pol III gene transcription in hepatocytes through MSK1. Our mechanism analysis has demonstrated that alcohol-caused high-response fragment of the Brf1 promoter is at p-382/+109bp. The MSK1 inhibitor SB-747651A is an effective reagent to repress alcohol-induced cell proliferation and colony formation, which is a potential pharmaceutical agent. Developing this inhibitor as a therapeutic approach will benefit alcohol-associated HCC patients.
Copyright © 2020 Mingen Lin et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

