Liver injury induced by paracetamol and challenges associated with intentional and unintentional use
- PMID: 32685105
- PMCID: PMC7336293
- DOI: 10.4254/wjh.v12.i4.125
Liver injury induced by paracetamol and challenges associated with intentional and unintentional use
Abstract
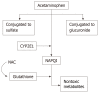
Drug induced liver injury (DILI) is a common cause of acute liver injury. Paracetamol, also known as acetaminophen, is a widely used anti-pyretic that has long been established to cause liver toxicity once above therapeutic levels. Hepatotoxicity from paracetamol overdose, whether intentional or non-intentional, is the most common cause of DILI in the United States and remains a global issue. Given the increased prevalence of combination medications in the form of pain relievers and antihistamines, paracetamol can be difficult to identify and remains a significant cause of acute hepatotoxicity, as evidenced by its contribution to over half of all acute liver failure cases in the United States. This is especially concerning given that, when co-ingested with other medications, the rise in serum paracetamol levels may be delayed past the 4-hour post-ingestion mark that is currently used to determine patients that require medical therapy. This review serves to describe the clinical and pathophysiologic features of hepatotoxicity secondary to paracetamol and provide an update on current available knowledge and treatment options.
Keywords: Acute liver failure; Drug-induced liver injury; Hepatotoxicity; Paracetamol.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Dr. Pyrsopoulos reports grants from Allergan, grants from Bayer, grants from Beigene, grants from Bristol Myers, grants from Confirm, grants from Conatus, grants from Intercept, grants from Mallinckrodt, grants from Novartis, grants from Resusix, grants from Saro, grants from Valeant, grants from Gilead, grants from Exelixis, grants from Hologic, grants from Shire, grants from Genfit, grants from Prometheus, outside the submitted work. Dr. Rotundo certifies that she has no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Figures

Similar articles
-
Hepatotoxicity of paracetamol and related fatalities.Eur Rev Med Pharmacol Sci. 2017 Mar;21(1 Suppl):95-101. Eur Rev Med Pharmacol Sci. 2017. PMID: 28379590
-
Accuracy of the paracetamol-aminotransferase multiplication product to predict hepatotoxicity in modified-release paracetamol overdose.Clin Toxicol (Phila). 2017 Jun;55(5):346-351. doi: 10.1080/15563650.2017.1290253. Epub 2017 Feb 15. Clin Toxicol (Phila). 2017. PMID: 28421844
-
Plasma paracetamol concentration at hospital presentation has a dose-dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine.Clin Toxicol (Phila). 2016 Jun;54(5):405-10. doi: 10.3109/15563650.2016.1159309. Clin Toxicol (Phila). 2016. PMID: 27108714
-
Acetaminophen: Dose-Dependent Drug Hepatotoxicity and Acute Liver Failure in Patients.Dig Dis. 2015;33(4):464-71. doi: 10.1159/000374090. Epub 2015 Jul 6. Dig Dis. 2015. PMID: 26159260 Free PMC article. Review.
-
Does cytochrome P450 liver isoenzyme induction increase the risk of liver toxicity after paracetamol overdose?Open Access Emerg Med. 2011 Oct 13;3:69-76. doi: 10.2147/OAEM.S24962. eCollection 2011. Open Access Emerg Med. 2011. PMID: 27147854 Free PMC article. Review.
Cited by
-
Paracetamol use during pregnancy - a call for precautionary action.Nat Rev Endocrinol. 2021 Dec;17(12):757-766. doi: 10.1038/s41574-021-00553-7. Epub 2021 Sep 23. Nat Rev Endocrinol. 2021. PMID: 34556849 Free PMC article. Review.
-
Toxic Epidermal Necrolysis Following the Ingestion of Paracetamol in a Pediatric Patient: A Case Report.Cureus. 2023 Nov 3;15(11):e48216. doi: 10.7759/cureus.48216. eCollection 2023 Nov. Cureus. 2023. PMID: 38050528 Free PMC article.
-
Transcriptomic signature, bioactivity and safety of a non-hepatotoxic analgesic generating AM404 in the midbrain PAG region.Sci Rep. 2024 May 15;14(1):11103. doi: 10.1038/s41598-024-61791-z. Sci Rep. 2024. PMID: 38750093 Free PMC article. Clinical Trial.
-
Modulation of Paracetamol-Induced Hepatotoxicity by Acute and Chronic Ethanol Consumption in Mice: A Study Pilot.Toxics. 2024 Nov 27;12(12):857. doi: 10.3390/toxics12120857. Toxics. 2024. PMID: 39771072 Free PMC article.
-
Evaluation of Nutraceutical Potential of Carduus marianus: Antioxidant and Hepatoprotective Effects in Paracetamol-Induced Hepatotoxicity and GC-MS Analysis.Food Sci Nutr. 2025 Jul 18;13(7):e70474. doi: 10.1002/fsn3.70474. eCollection 2025 Jul. Food Sci Nutr. 2025. PMID: 40688605 Free PMC article.
References
-
- Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–2534. - PubMed
-
- Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102:2459–2463. - PubMed
-
- Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296:87–93. - PubMed
-
- Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM U. S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

