A little frog leaps a long way: compounded colonizations of the Indian Subcontinent discovered in the tiny Oriental frog genus Microhyla (Amphibia: Microhylidae)
- PMID: 32685285
- PMCID: PMC7337035
- DOI: 10.7717/peerj.9411
A little frog leaps a long way: compounded colonizations of the Indian Subcontinent discovered in the tiny Oriental frog genus Microhyla (Amphibia: Microhylidae)
Abstract
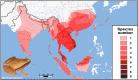
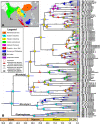
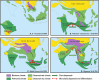
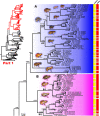
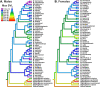
Frogs of the genus Microhyla include some of the world's smallest amphibians and represent the largest radiation of Asian microhylids, currently encompassing 50 species, distributed across the Oriental biogeographic region. The genus Microhyla remains one of the taxonomically most challenging groups of Asian frogs and was found to be paraphyletic with respect to large-sized fossorial Glyphoglossus. In this study we present a time-calibrated phylogeny for frogs in the genus Microhyla, and discuss taxonomy, historical biogeography, and morphological evolution of these frogs. Our updated phylogeny of the genus with nearly complete taxon sampling includes 48 nominal Microhyla species and several undescribed candidate species. Phylogenetic analyses of 3,207 bp of combined mtDNA and nuDNA data recovered three well-supported groups: the Glyphoglossus clade, Southeast Asian Microhyla II clade (includes M. annectens species group), and a diverse Microhyla I clade including all other species. Within the largest major clade of Microhyla are seven well-supported subclades that we identify as the M. achatina, M. fissipes, M. berdmorei, M. superciliaris, M. ornata, M. butleri, and M. palmipes species groups. The phylogenetic position of 12 poorly known Microhyla species is clarified for the first time. These phylogenetic results, along with molecular clock and ancestral area analyses, show the Microhyla-Glyphoglossus assemblage to have originated in Southeast Asia in the middle Eocene just after the first hypothesized land connections between the Indian Plate and the Asian mainland. While Glyphoglossus and Microhyla II remained within their ancestral ranges, Microhyla I expanded its distribution generally east to west, colonizing and diversifying through the Cenozoic. The Indian Subcontinent was colonized by members of five Microhyla species groups independently, starting with the end Oligocene-early Miocene that coincides with an onset of seasonally dry climates in South Asia. Body size evolution modeling suggests that four groups of Microhyla have independently achieved extreme miniaturization with adult body size below 15 mm. Three of the five smallest Microhyla species are obligate phytotelm-breeders and we argue that their peculiar reproductive biology may be a factor involved in miniaturization. Body size increases in Microhyla-Glyphoglossus seem to be associated with a burrowing adaptation to seasonally dry habitats. Species delimitation analyses suggest a vast underestimation of species richness and diversity in Microhyla and reveal 15-33 undescribed species. We revalidate M. nepenthicola, synonymize M. pulverata with M. marmorata, and provide insights on taxonomic statuses of a number of poorly known species. Further integrative studies, combining evidence from phylogeny, morphology, advertisement calls, and behavior will result in a better systematic understanding of this morphologically cryptic radiation of Asian frogs.
Keywords: Biogeography; Cryptic species; Glyphoglossus; Indian collision; Microhylinae; Miniaturization; Molecular phylogeny; Narrow-mouthed frogs; Southeast Asia; Species delimitation.
© 2020 Gorin et al.
Conflict of interest statement
Nikolay A. Poyarkov is an Academic Editor for PeerJ. Suranjan Karunarathna is employed by Nature Explorations and Education Team (Sri Lanka). Luan Thanh Nguyen is employed by Asian Turtle Program—Indo-Myanmar Conservation (Vietnam). Other authors have declared that no competing interests exist.
Figures


References
-
- Acton GD. Apparent polar wander of India since the Cretaceous with implications for regional tectonics and true polar wander. In: Radhakrishna T, Piper JDA, editors. The Indian Subcontinent and Gondwana: a Palaeomagnetic and Rock Magnetic Perspective. Vol. 44. Bangalore: Memoires of Geological Society of India; 1999. pp. 129–175.
-
- Agarwal I, Biswas S, Bauer AM, Greenbaum E, Jackman TR, De Silva A, Batuwita S. Cryptic species, taxonomic inflation, or a bit of both? New species phenomenon in Sri Lanka as suggested by a phylogeny of dwarf geckos (Reptilia, Squamata, Gekkonidae, Cnemaspis) Systematics and Biodiversity. 2017;15(5):1–13. doi: 10.1080/14772000.2017.1282553. - DOI
-
- Aitchison JC, Ali JR, Davis AM. When and where did India and Asia collide? Journal of Geophysical Research: Solid Earth. 2007;112:B05423.
-
- Ali JR, Aitchison JC. Gondwana to Asia: plate tectonics, paleogeography and the biological connectivity of the Indian subcontinent from the Middle Jurassic through latest Eocene (166-35 Ma) Earth-Science Reviews. 2008;88(3–4):145–166. doi: 10.1016/j.earscirev.2008.01.007. - DOI
LinkOut - more resources
Full Text Sources

