Endo-chitinase Chit33 specificity on different chitinolytic materials allows the production of unexplored chitooligosaccharides with antioxidant activity
- PMID: 32685384
- PMCID: PMC7355052
- DOI: 10.1016/j.btre.2020.e00500
Endo-chitinase Chit33 specificity on different chitinolytic materials allows the production of unexplored chitooligosaccharides with antioxidant activity
Abstract
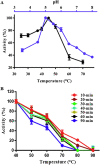
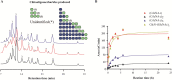
The biological activity of chitooligosaccharides (COS) has made them targets for industrial and medical sectors. In this work, endo-chitinase Chit33 from Trichoderma harzianum CECT 2413 was expressed in Pichia pastoris GS115 to levels never achieved before (630 mg/L; 3.3 U/mL), without its biochemical characteristics being substantially affected. Chit33 produced a mixture of fully and partially acetylated COS from different chitin derivatives. HPAEC-PAD Chromatography and mass spectrometry analyses showed that (GlcNAc)4 and GlcN-(GlcNAc)2 were mainly produced from colloidal chitin and chitosan, respectively. COS in reaction mixtures were fragmented according to their size and their antioxidant activity analyzed by reducing power and free radical scavenging activity essays. The highest antioxidant activity was achieved with COS in the range of 0.5-2 and 2-10 kDa produced from colloidal chitin and chitosan, respectively, which gives biotechnological potential to both the chitin derivatives of 0.5-10 kDa and the biocatalyst producing them.
Keywords: Bioactive chito-oligomers; COS, chitooligosaccharides; Chitin; Chitosan; DD, degree of deacetylation; DP, degree of polymerization; FRAP, Ferric Reducing Antioxidant Power; GH, Glycoside Hydrolase; GlcN, D-glucosamine; GlcNAc, N-Acetyl-d-glucosamine; Heterologous expression; Pichia pastoris; RSA, Radical Scavenging Activity; paCOS, partially acetylated COS.
© 2020 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors do not have any conflict of interest.
Figures




Similar articles
-
Chitinous material bioconversion by three new chitinases from the yeast Mestchnikowia pulcherrima.Microb Cell Fact. 2024 Jan 20;23(1):31. doi: 10.1186/s12934-024-02300-9. Microb Cell Fact. 2024. PMID: 38245740 Free PMC article. Review.
-
Use of chitin and chitosan to produce new chitooligosaccharides by chitinase Chit42: enzymatic activity and structural basis of protein specificity.Microb Cell Fact. 2018 Mar 22;17(1):47. doi: 10.1186/s12934-018-0895-x. Microb Cell Fact. 2018. PMID: 29566690 Free PMC article.
-
Boosting Biocatalytic Efficiency: Engineering of Chitinase Chit33 with Chitin and Cellulose Binding Domains for Sustainable Chitin Conversion.J Agric Food Chem. 2025 May 7;73(18):11121-11131. doi: 10.1021/acs.jafc.4c10364. Epub 2025 Apr 25. J Agric Food Chem. 2025. PMID: 40279401 Free PMC article.
-
Enzymatic single-step preparation and antioxidant activity of hetero-chitooligosaccharides using non-pretreated housefly larvae powder.Carbohydr Polym. 2017 Sep 15;172:113-119. doi: 10.1016/j.carbpol.2017.05.037. Epub 2017 May 12. Carbohydr Polym. 2017. PMID: 28606517
-
The cell factory approach toward biotechnological production of high-value chitosan oligomers and their derivatives: an update.Crit Rev Biotechnol. 2017 Feb;37(1):11-25. doi: 10.3109/07388551.2015.1104289. Epub 2015 Nov 2. Crit Rev Biotechnol. 2017. PMID: 26526199 Review.
Cited by
-
Recombinant expression and characterization of the endochitinase Chit36-TA from Trichoderma asperellum in Komagataella phaffii for chitin degradation of black soldier fly exuviae.Bioprocess Biosyst Eng. 2024 Oct;47(10):1751-1766. doi: 10.1007/s00449-024-03067-4. Epub 2024 Aug 8. Bioprocess Biosyst Eng. 2024. PMID: 39115691 Free PMC article.
-
Chitinous material bioconversion by three new chitinases from the yeast Mestchnikowia pulcherrima.Microb Cell Fact. 2024 Jan 20;23(1):31. doi: 10.1186/s12934-024-02300-9. Microb Cell Fact. 2024. PMID: 38245740 Free PMC article. Review.
-
Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects.Trends Food Sci Technol. 2020 Nov;105:17-42. doi: 10.1016/j.tifs.2020.08.016. Epub 2020 Sep 4. Trends Food Sci Technol. 2020. PMID: 32901176 Free PMC article. Review.
-
A Novel Porous Butyryl Chitin-Animal Derived Hydroxyapatite Composite Scaffold for Cranial Bone Defect Repair.Int J Mol Sci. 2023 May 10;24(10):8519. doi: 10.3390/ijms24108519. Int J Mol Sci. 2023. PMID: 37239867 Free PMC article.
-
Activity of a Recombinant Chitinase of the Atta sexdens Ant on Different Forms of Chitin and Its Fungicidal Effect against Lasiodiplodia theobromae.Polymers (Basel). 2024 Feb 15;16(4):529. doi: 10.3390/polym16040529. Polymers (Basel). 2024. PMID: 38399907 Free PMC article.
References
-
- Deng J.J., Shi D., hua Mao H., wei Li Z., Liang S., Ke Y., chun Luo X. Heterologous expression and characterization of an antifungal chitinase (Chit46) from Trichoderma harzianum GIM 3.442 and its application in colloidal chitin conversion. Int. J. Biol. Macromol. 2019;134:113–121. doi: 10.1016/j.ijbiomac.2019.04.177. - DOI - PubMed
-
- Khalil A.M., Abdel-Monem R.A., Darwesh O.M., Hashim A.I., Nada A.A., Rabie S.T. Synthesis, characterization, and evaluation of antimicrobial activities of chitosan and carboxymethyl chitosan Schiff-base/silver nanoparticles. J. Chem. 2017:1–12. doi: 10.1155/2017/1434320. - DOI
-
- Hamed I., Özogul F., Regenstein J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci. Technol. 2016;48:40–50. doi: 10.1016/j.tifs.2015.11.007. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
