Pathophysiological Changes in Female Rats with Estrous Cycle Disorder Induced by Long-Term Heat Stress
- PMID: 32685488
- PMCID: PMC7320282
- DOI: 10.1155/2020/4701563
Pathophysiological Changes in Female Rats with Estrous Cycle Disorder Induced by Long-Term Heat Stress
Abstract
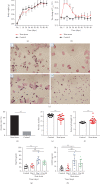
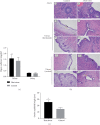
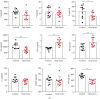
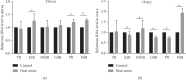
High-temperature exposure is detrimental to women's reproductive health; however, the impact caused by long-term high temperature is not comprehensive, and a stable model of estrous cycle disorder induced by a high temperature is yet lacking. Herein, we aimed to establish a stable and effective model of estrous cycle disorder in female rats induced by long-term heat stress to study its physiological and pathological characteristics and explore the underlying mechanism. In the present study, female Sprague-Dawley rats with normal estrous cycles were exposed to the temperature of 38 ± 0.5°C, relative humidity (RH) of 55 ± 5% (2 h/d, 1 time/d) hot cabin at more than 90 days. Consequently, after long-term heat stress, no difference was detected in body weight and rectal temperature, but the estrus cycle was prolonged, the uterine organ index was increased, pathological changes occurred, the increase latitude of stress hormones heat shock protein 70 (Hsp70) and corticosterone (CORT) decreased, estradiol (E2) and luteinizing hormone (LH) levels decreased, follicle stimulating hormone (FSH) and prolactin (Prl) levels increased, gonadotropin-releasing hormone (GnRH) and thyroid hormone (T4) showed no difference, and insulin (INS) decreased significantly. Moreover, the mRNA expression of the sex hormone receptor in the uterus and ovary was altered. Therefore, the estrous cycle disorder in female rats can be induced by regular heat stress for 90 days, which can be considered the pioneer method. Subsequently, prominent physiological and pathological characteristics and disruption in the hypothalamic-pituitary-gonadal (HPG) axis were noted.
Copyright © 2020 GaiHong An et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Sils I. V., Matthew C. B., Bastille A. M. Estrus related differences in response to a hot environment in telemetry- equipped female rats. Journal of Thermal Biology. 2002;27(4):279–284. doi: 10.1016/S0306-4565(01)00020-1. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

