Biochemical Characterization of Lactose Binding Entadin Lectin from Entada rheedii Seeds with Cytotoxic Activity against Cancer Cell Lines
- PMID: 32685806
- PMCID: PMC7366353
- DOI: 10.1021/acsomega.0c00577
Biochemical Characterization of Lactose Binding Entadin Lectin from Entada rheedii Seeds with Cytotoxic Activity against Cancer Cell Lines
Abstract
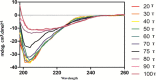
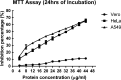
A novel Entadin lectin was isolated, purified, and characterized from the seeds of Entada rheedii by ammonium sulfate precipitation, followed by lactose affinity chromatography. On sodium dodecyl sulfate polyacrylamide gel electrophoresis, the purified Entadin lectin appeared as a single band (monomeric in nature) with a molecular mass of approximately 20 kDa in both reducing and nonreducing conditions. Mass spectroscopic analysis confirms the molecular weight of Entadin lectin as 19,333 Da. Entadin lectin showed a highest titer value in agglutination against human blood group B red blood cells, and its hemagglutination activity was inhibited by lactose, cellobiose, and galactose. Periodic acid Schiff staining confirmed the glycoprotein nature of Entadin lectin with an approximately 5% carbohydrate content. This lectin is highly stable even after incubation at a wide range of temperatures (30-60 °C) and pHs (6-10). The antiproliferative effect of Entadin lectin against lung cancer cells A549 and cervical cancer cells HeLa showed IC50 values of 28 and 32 μg/mL, respectively, and no antiproliferative activity against normal cells was observed. Cell morphological studies revealed that Entadin lectin induced apoptosis in both A549 and HeLa cancer cells, which was confirmed by acridine orange/ethidium bromide and Hoechst (33258) nuclear counterstaining.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- de Oliveira Figueiroa E.; Albuquerque da Cunha C. R.; Albuquerque P. B. S.; de Paula R. A.; Aranda-Souza M. A.; Alves M. S.; Zagmignan A.; Carneiro-da-Cunha M. G.; Nascimento da Silva L. C.; Dos Santos Correia M. T. Lectin-Carbohydrate Interactions: Implications for the Development of New Anticancer Agents. Curr. Med. Chem. 2017, 24, 3667–3680. 10.2174/0929867324666170523110400. - DOI - PubMed
-
- Cavada B. S.; Silva M. T. L.; Osterne V. J. S.; Pinto-Junior V. R.; Nascimento A. P. M.; Wolin I. A. V.; Heinrich I. A.; Nobre C. A. S.; Moreira C. G.; Lossio C. F.; Rocha C. R. C.; Martins J. L.; Nascimento K. S.; Leal R. B. Canavalia bonariensis lectin: Molecular bases of glycoconjugates interaction and antiglioma potential. Int. J. Biol. Macromol. 2018, 106, 369–378. 10.1016/j.ijbiomac.2017.08.023. - DOI - PubMed
LinkOut - more resources
Full Text Sources

