Antiviral anticoagulation
- PMID: 32685886
- PMCID: PMC7354393
- DOI: 10.1002/rth2.12406
Antiviral anticoagulation
Abstract
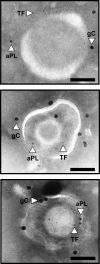
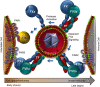
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel envelope virus that causes coronavirus disease 2019 (COVID-19). Hallmarks of COVID-19 are a puzzling form of thrombophilia that has elevated D-dimer but only modest effects on other parameters of coagulopathy. This is combined with severe inflammation, often leading to acute respiratory distress and possible lethality. Coagulopathy and inflammation are interconnected by the transmembrane receptor, tissue factor (TF), which initiates blood clotting as a cofactor for factor VIIa (FVIIa)-mediated factor Xa (FXa) generation. TF also functions from within the nascent TF/FVIIa/FXa complex to trigger profound changes via protease-activated receptors (PARs) in many cell types, including SARS-CoV-2-trophic cells. Therefore, aberrant expression of TF may be the underlying basis of COVID-19 symptoms. Evidence suggests a correlation between infection with many virus types and development of clotting-related symptoms, ranging from heart disease to bleeding, depending on the virus. Since numerous cell types express TF and can act as sites for virus replication, a model envelope virus, herpes simplex virus type 1 (HSV1), has been used to investigate the uptake of TF into the envelope. Indeed, HSV1 and other viruses harbor surface TF antigen, which retains clotting and PAR signaling function. Strikingly, envelope TF is essential for HSV1 infection in mice, and the FXa-directed oral anticoagulant apixaban had remarkable antiviral efficacy. SARS-CoV-2 replicates in TF-bearing epithelial and endothelial cells and may stimulate and integrate host cell TF, like HSV1 and other known coagulopathic viruses. Combined with this possibility, the features of COVID-19 suggest that it is a TFopathy, and the TF/FVIIa/FXa complex is a feasible therapeutic target.
Keywords: COVID‐19; coagulation; herpes simplex virus; inflammation; protease‐activated receptor; tissue factor.
© 2020 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Figures


References
-
- Luo W, Yu H, Guo J, Li X, Sun Y, Li J, Liu L. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID‐19). Pre‐prints. 2020. 2020020407.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

