The choroid plexus stroma constitutes a sanctuary for paediatric B-cell precursor acute lymphoblastic leukaemia in the central nervous system
- PMID: 32686161
- PMCID: PMC7540040
- DOI: 10.1002/path.5510
The choroid plexus stroma constitutes a sanctuary for paediatric B-cell precursor acute lymphoblastic leukaemia in the central nervous system
Abstract
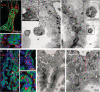
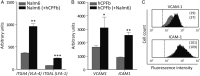
Despite current central nervous system-directed therapies for childhood B-cell precursor acute lymphoblastic leukaemia, relapse at this anatomical site still remains a challenging issue. Few reports have addressed the study of the specific cellular microenvironments which can promote the survival, quiescence, and therefore chemoresistance of B-cell precursor acute lymphoblastic leukaemia cells in the central nervous system. Herein, we showed by immunofluorescence and electron microscopy that in xenotransplanted mice, leukaemic cells infiltrate the connective tissue stroma of the choroid plexus, the brain structure responsible for the production of cerebrospinal fluid. The ultrastructural study also showed that leukaemia cells are able to migrate through blood vessels located in the choroid plexus stroma. In short-term co-cultures, leukaemic cells established strong interactions with human choroid plexus fibroblasts, mediated by an increased expression of ITGA4 (VLA-4)/ITGAL (LFA-1) and their ligands VCAM1/ICAM1. Upon contact with leukaemia cells, human choroid plexus fibroblasts acquired a cancer-associated fibroblast phenotype, with an increased expression of α-SMA and vimentin as well as pro-inflammatory factors. Human choroid plexus fibroblasts also have the capacity to reduce the proliferative index of leukaemic blasts and promote their survival and chemoresistance to methotrexate and cytarabine. The inhibition of VLA-4/VCAM-1 interactions using anti-VLA-4 antibodies, and the blockade of Notch signalling pathway by using a γ-secretase inhibitor partially restored chemotherapy sensitivity of leukaemia cells. We propose that the choroid plexus stroma constitutes a sanctuary for B-cell precursor acute lymphoblastic leukaemia cells in the central nervous system. © 2020 The Authors. The Journal of Pathology published by John Wiley & Sons, Ltd. on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: central nervous system; choroid plexus; haematopoiesis; lymphocytes; paediatric B-cell precursor acute lymphoblastic leukaemia.
© 2020 The Authors. The Journal of Pathology published by John Wiley & Sons, Ltd. on behalf of The Pathological Society of Great Britain and Ireland.
Figures




References
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med 2015; 373 : 1541–1552. - PubMed
-
- Ward E, DeSantis C, Robbins A, et al Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014; 64 : 83–103. - PubMed
-
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet 2008; 371 : 1030–1043. - PubMed
-
- Thomas LB. Pathology of leukemia in the brain and meninges: postmortem studies of patients with acute leukemia and of mice given inoculations of L1210 leukemia. Cancer Res 1965; 25 : 1555–1571. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

