Identification of a new strain of mouse kidney parvovirus associated with inclusion body nephropathy in immunocompromised laboratory mice
- PMID: 32686622
- PMCID: PMC7473309
- DOI: 10.1080/22221751.2020.1798288
Identification of a new strain of mouse kidney parvovirus associated with inclusion body nephropathy in immunocompromised laboratory mice
Abstract
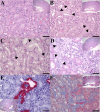
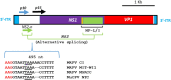
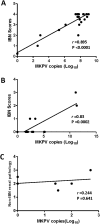
Inclusion body nephropathy (IBN) and kidney fibrosis in aged immunodeficient mice and, to lesser extent, in immunocompetent mice have been recently linked to infection of mouse kidney parvovirus (MKPV), also known as murine chapparvovirus (MuCPV). Knowledge about its prevalence and the complete genome sequence of more MKPV strains is essential for understanding phylogenetic relationships and pathogenicity among MKPV strains. In the present study using PCR and genome walking, we determined the complete 4440-nucleotide genome of a new MKPV strain, namely MIT-WI1, which was identified in IBN-affected Il2rg-/-Rag2-/- c-Kit W-sh/W-sh mice housed in the vivarium at Whitehead Institute for Biomedical Research (WI). The overall nucleotide (>94%) and deduced amino acid sequences (>98%) of p10, p15, NS1 (replicase), NS2 and VP1 (capsid protein) within the MIT-WI1 genome, are closely related to MKPV/MuCPV strains described in laboratory and wild Mus musculus mice. In addition, PCR and qPCR assays using newly designed primers conserved among the known MKPV/MuCPV genomes were developed and utilized to assess MKPV status in selected laboratory mice. MKPV was also detected in immunodeficient (NSG) and immunocompetent (Crl:CD1(ICR), UTXflox) mouse strains/stocks. The abundance of the MKPV genome copies was significantly correlated with the severity of IBN. Our data indicate that MKPV is present in selected mouse strains/stocks, and provides new insights into the genome evolution of MKPV.
Keywords: IBN; MKPV; PCR diagnosis; genome organization.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Barthold SW, Percy DH, Griffey SM.. Pathology of laboratory rodents and rabbits. 4th ed. Hoboken, NJ: John Wiley & Sons; 2016.
-
- Baze WB, Steinbach TJ, Fleetwood ML, et al. . Karyomegaly and intranuclear inclusions in the renal tubules of sentinel ICR mice (mus musculus). Comp Med. 2006 Oct;56(5):435–438. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
