Basement membrane damage by ROS- and JNK-mediated Mmp2 activation drives macrophage recruitment to overgrown tissue
- PMID: 32686670
- PMCID: PMC7371875
- DOI: 10.1038/s41467-020-17399-8
Basement membrane damage by ROS- and JNK-mediated Mmp2 activation drives macrophage recruitment to overgrown tissue
Abstract
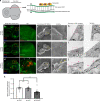
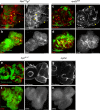
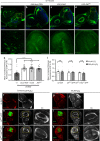
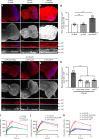
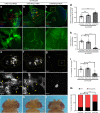
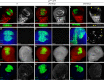
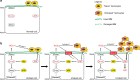
Macrophages are a major immune cell type infiltrating tumors and promoting tumor growth and metastasis. To elucidate the mechanism of macrophage recruitment, we utilize an overgrowth tumor model ("undead" model) in larval Drosophila imaginal discs that are attached by numerous macrophages. Here we report that changes to the microenvironment of the overgrown tissue are important for recruiting macrophages. First, we describe a correlation between generation of reactive oxygen species (ROS) and damage of the basement membrane (BM) in all neoplastic, but not hyperplastic, models examined. ROS and the stress kinase JNK mediate the accumulation of matrix metalloproteinase 2 (Mmp2), damaging the BM, which recruits macrophages to the tissue. We propose a model where macrophage recruitment to and activation at overgrowing tissue is a multi-step process requiring ROS- and JNK-mediated Mmp2 upregulation and BM damage. These findings have implications for understanding the role of the tumor microenvironment for macrophage activation.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Cap-n-Collar Promotes Tissue Regeneration by Regulating ROS and JNK Signaling in the Drosophila melanogaster Wing Imaginal Disc.Genetics. 2017 Jul;206(3):1505-1520. doi: 10.1534/genetics.116.196832. Epub 2017 May 16. Genetics. 2017. PMID: 28512185 Free PMC article.
-
Extracellular Reactive Oxygen Species Drive Apoptosis-Induced Proliferation via Drosophila Macrophages.Curr Biol. 2016 Mar 7;26(5):575-84. doi: 10.1016/j.cub.2015.12.064. Epub 2016 Feb 18. Curr Biol. 2016. PMID: 26898463 Free PMC article.
-
The beneficial role of extracellular reactive oxygen species in apoptosis-induced compensatory proliferation.Fly (Austin). 2017 Jan 2;11(1):46-52. doi: 10.1080/19336934.2016.1222997. Epub 2016 Aug 15. Fly (Austin). 2017. PMID: 27575697 Free PMC article. Review.
-
Tumor-promoting function of apoptotic caspases by an amplification loop involving ROS, macrophages and JNK in Drosophila.Elife. 2017 Aug 30;6:e26747. doi: 10.7554/eLife.26747. Elife. 2017. PMID: 28853394 Free PMC article.
-
Regeneration and transdetermination in Drosophila imaginal discs.Annu Rev Genet. 2012;46:289-310. doi: 10.1146/annurev-genet-110711-155637. Epub 2012 Aug 29. Annu Rev Genet. 2012. PMID: 22934642 Review.
Cited by
-
Subtyping of gastric cancer based on basement membrane genes that stratifies the prognosis, immune infiltration and therapeutic response.Discov Oncol. 2024 Aug 20;15(1):362. doi: 10.1007/s12672-024-01238-z. Discov Oncol. 2024. PMID: 39164593 Free PMC article.
-
Anti-Tumor Effect of Turandot Proteins Induced via the JAK/STAT Pathway in the mxc Hematopoietic Tumor Mutant in Drosophila.Cells. 2023 Aug 11;12(16):2047. doi: 10.3390/cells12162047. Cells. 2023. PMID: 37626857 Free PMC article.
-
Identification and Validation of Basement Membrane Related LncRNA Signatures as a Novel Prognostic Model for Hepatocellular Carcinoma.Biochem Genet. 2025 Jun;63(3):2100-2119. doi: 10.1007/s10528-024-10797-3. Epub 2024 Apr 29. Biochem Genet. 2025. PMID: 38684626 Free PMC article.
-
Study on the In Vitro and In Vivo Antioxidant Activity and Potential Mechanism of Polygonum viviparum L.Antioxidants (Basel). 2025 Jan 1;14(1):41. doi: 10.3390/antiox14010041. Antioxidants (Basel). 2025. PMID: 39857375 Free PMC article.
-
Identification of Fibrotic Biomarkers Associated with Macrophages in Diabetic Nephropathy.Med Sci Monit. 2023 Nov 15;29:e940847. doi: 10.12659/MSM.940847. Med Sci Monit. 2023. PMID: 37964514 Free PMC article.
References
-
- Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. - PubMed
-
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013;229:176–185. - PubMed
-
- Goswami KK, et al. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2017;316:1–10. - PubMed
-
- Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

