Mutations in COMP cause familial carpal tunnel syndrome
- PMID: 32686688
- PMCID: PMC7371736
- DOI: 10.1038/s41467-020-17378-z
Mutations in COMP cause familial carpal tunnel syndrome
Erratum in
-
Author Correction: Mutations in COMP cause familial carpal tunnel syndrome.Nat Commun. 2020 Aug 3;11(1):3931. doi: 10.1038/s41467-020-17845-7. Nat Commun. 2020. PMID: 32747625 Free PMC article.
Abstract
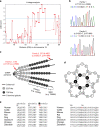
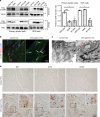
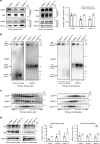
Carpal tunnel syndrome (CTS) is the most common peripheral nerve entrapment syndrome, affecting a large proportion of the general population. Genetic susceptibility has been implicated in CTS, but the causative genes remain elusive. Here, we report the identification of two mutations in cartilage oligomeric matrix protein (COMP) that segregate with CTS in two large families with or without multiple epiphyseal dysplasia (MED). Both mutations impair the secretion of COMP by tenocytes, but the mutation associated with MED also perturbs its secretion in chondrocytes. Further functional characterization of the CTS-specific mutation reveals similar histological and molecular changes of tendons/ligaments in patients' biopsies and the mouse models. The mutant COMP fails to oligomerize properly and is trapped in the ER, resulting in ER stress-induced unfolded protein response and cell death, leading to inflammation, progressive fibrosis and cell composition change in tendons/ligaments. The extracellular matrix (ECM) organization is also altered. Our studies uncover a previously unrecognized mechanism in CTS pathogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Padua L, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. 2016;15:1273–1284. - PubMed
-
- Cobb TK, Bond JR, Cooney WP, Metcalf BJ. Assessment of the ratio of carpal contents to carpal tunnel volume in patients with carpal tunnel syndrome: a preliminary report. J. Hand Surg. Am. 1997;22:635–639. - PubMed
-
- Sernik RA, et al. Ultrasound features of carpal tunnel syndrome: a prospective case-control study. Skelet. Radio. 2008;37:49–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

