A Phase IIa Controlled Human Malaria Infection and Immunogenicity Study of RTS,S/AS01E and RTS,S/AS01B Delayed Fractional Dose Regimens in Malaria-Naive Adults
- PMID: 32687161
- PMCID: PMC7552430
- DOI: 10.1093/infdis/jiaa421
A Phase IIa Controlled Human Malaria Infection and Immunogenicity Study of RTS,S/AS01E and RTS,S/AS01B Delayed Fractional Dose Regimens in Malaria-Naive Adults
Abstract
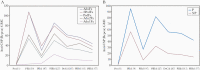
Background: A previous RTS,S/AS01B vaccine challenge trial demonstrated that a 3-dose (0-1-7-month) regimen with a fractional third dose can produce high vaccine efficacy (VE) in adults challenged 3 weeks after vaccination. This study explored the VE of different delayed fractional dose regimens of adult and pediatric RTS,S/AS01 formulations.
Methods: A total of 130 participants were randomized into 5 groups. Four groups received 3 doses of RTS,S/AS01B or RTS,S/AS01E on a 0-1-7-month schedule, with the final 1 or 2 doses being fractional (one-fifth dose volume). One group received 1 full (month 0) and 1 fractional (month 7) dose of RTS,S/AS01E. Immunized and unvaccinated control participants underwent Plasmodium falciparum-infected mosquito challenge (controlled human malaria infection) 3 months after immunization, a timing chosen to potentially discriminate VEs between groups.
Results: The VE of 3-dose formulations ranged from 55% (95% confidence interval, 27%-72%) to 76% (48%-89%). Groups administered equivalent formulations of RTS,S/AS01E and RTS,S/AS01B demonstrated comparable VE. The 2-dose group demonstrated lower VE (29% [95% confidence interval, 6%-46%]). All regimens were well tolerated and immunogenic, with trends toward higher anti-circumsporozoite antibody titers in participants protected against infection.
Conclusions: RTS,S/AS01E can provide VE comparable to an equivalent RTS,S/AS01B regimen in adults, suggesting a universal formulation may be considered. Results also suggest that the 2-dose regimen is inferior to the 3-dose regimens evaluated.
Clinical trial registration: NCT03162614.
Keywords: Plasmodium falciparum; 3-month challenge; RTS; S/AS01; controlled human malaria infection; delayed fractional dose; efficacy; immunogenicity; malaria; safety.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



Comment in
-
Optimizing RTS,S Vaccination Strategies: Give It Your Best Parting Shot.J Infect Dis. 2020 Oct 13;222(10):1581-1584. doi: 10.1093/infdis/jiaa423. J Infect Dis. 2020. PMID: 32685977 No abstract available.
References
-
- World Health Organization–Malaria Vaccine Funders Group. 2013 Update to the Malaria Vaccine Technology Roadmap. 2013. Available at: https://www.who.int/immunization/sage/meetings/2013/april/6_Draft_roadma.... Accessed 28 January 2020.
-
- RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863–75. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

