Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections
- PMID: 32687484
- PMCID: PMC7526441
- DOI: 10.1172/jci.insight.140329
Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections
Abstract
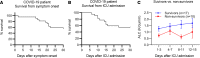
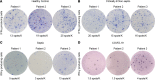
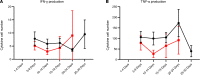
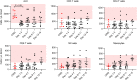
COVID-19-associated morbidity and mortality have been attributed to a pathologic host response. Two divergent hypotheses have been proposed: hyperinflammatory cytokine storm; and failure of host protective immunity that results in unrestrained viral dissemination and organ injury. A key explanation for the inability to address this controversy has been the lack of diagnostic tools to evaluate immune function in COVID-19 infections. ELISpot, a highly sensitive, functional immunoassay, was employed in 27 patients with COVID-19, 51 patients with sepsis, 18 critically ill nonseptic (CINS) patients, and 27 healthy control volunteers to evaluate adaptive and innate immune status by quantitating T cell IFN-ɣ and monocyte TFN-α production. Circulating T cell subsets were profoundly reduced in COVID-19 patients. Additionally, stimulated blood mononuclear cells produced less than 40%-50% of the IFN-ɣ and TNF-α observed in septic and CINS patients, consistent with markedly impaired immune effector cell function. Approximately 25% of COVID-19 patients had increased IL-6 levels that were not associated with elevations in other canonical proinflammatory cytokines. Collectively, these findings support the hypothesis that COVID-19 suppresses host functional adaptive and innate immunity. Importantly, IL-7 administered ex vivo restored T cell IFN-ɣ production in COVID-19 patients. Thus, ELISpot may functionally characterize host immunity in COVID-19 and inform prospective therapies.
Keywords: Adaptive immunity; COVID-19.
Conflict of interest statement
Figures




References
-
- Sarzi-Puttini P, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

