Hemolytic disease of the fetus and newborn due to Rh(D) incompatibility: A preventable disease that still produces significant morbidity and mortality in children
- PMID: 32687543
- PMCID: PMC7371205
- DOI: 10.1371/journal.pone.0235807
Hemolytic disease of the fetus and newborn due to Rh(D) incompatibility: A preventable disease that still produces significant morbidity and mortality in children
Abstract
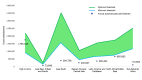
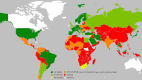
In the mid-20th century, Hemolytic Disease of the Fetus and Newborn, caused by maternal alloimmunization to the Rh(D) blood group antigen expressed by fetal red blood cells (i.e., "Rh disease"), was a major cause of fetal and neonatal morbidity and mortality. However, with the regulatory approval, in 1968, of IgG anti-Rh(D) immunoprophylaxis to prevent maternal sensitization, the prospect of eradicating Rh disease was at hand. Indeed, the combination of antenatal and post-partum immunoprophylaxis is ~99% effective at preventing maternal sensitization to Rh(D). To investigate global compliance with this therapeutic intervention, we used an epidemiological approach to estimate the current annual number of pregnancies worldwide involving an Rh(D)-negative mother and an Rh(D)-positive fetus. The annual number of doses of anti-Rh(D) IgG required for successful immunoprophylaxis for these cases was then calculated and compared with an estimate of the annual number of doses of anti-Rh(D) produced and provided worldwide. Our results suggest that ~50% of the women around the world who require this type of immunoprophylaxis do not receive it, presumably due to a lack of awareness, availability, and/or affordability, thereby putting hundreds of thousands of fetuses and neonates at risk for Rh disease each year. The global failure to provide this generally acknowledged standard-of-care to prevent Rh disease, even 50 years after its availability, contributes to an enormous, continuing burden of fetal and neonatal disease and provides a critically important challenge to the international health care system.
Conflict of interest statement
Dr. Spitalnik has read the journal's policy and the authors of this manuscript have the following competing interests: he is a member of the Scientific Advisory Board of Hemanext, Inc. and a consultant for Tioma, Inc. However, neither of these competing interests are relevant to the content of the submitted manuscript. Valeria Pegoraro and Duccio Urbinati are employees of IQVIA Solutions Italy S.r.l. However, none of these competing interests are relevant to the content of the submitted manuscript. The other authors have declared that no competing interests exist.
Figures
References
-
- Pollack W, Gorman JG, Freda VJ, Ascari WQ, Allen AE, Baker WJ. Results of clinical trials of RhoGAM in women. Transfusion 1968. May-Jun;9(3):151–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

