Utilization of the Thorpe-Ingold Effect in the Synthesis of Cyclooctanoid Ring Systems via Anionic 6- exo- dig Cyclization/Claisen Rearrangement Sequence
- PMID: 32687712
- PMCID: PMC8588887
- DOI: 10.1021/acs.joc.0c01132
Utilization of the Thorpe-Ingold Effect in the Synthesis of Cyclooctanoid Ring Systems via Anionic 6- exo- dig Cyclization/Claisen Rearrangement Sequence
Abstract
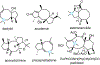
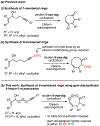
We demonstrate a facile approach for the synthesis of gem-disubstituted cyclooctanoids, a motif found in several biologically active compounds. Appropriately substituted 1-alkenyl-5-pentyn-1-ols bearing gem-dialkyl substituents at either the C2, C3, or C4 position serve as useful precursors to a number of cyclooct-4-enone derivatives via a tandem, microwave-assisted oxyanionic 6-exo-dig cyclization/Claisen rearrangement reaction. gem-Dialkyl activation is necessary for these reactions to occur, as unactivated 1-alkenyl-5-pentyn-1-ols fail to undergo 6-exo-dig cyclization under the conditions employed. Further application of the methodology to the corresponding gem-dialkoxy system was also explored to facilitate access to more complex carbocycles.
Figures


Similar articles
-
Synthesis of seven-membered carbocyclic rings via a microwave-assisted tandem oxyanionic 5-exo dig cyclization-claisen rearrangement process.J Org Chem. 2007 Aug 17;72(17):6624-7. doi: 10.1021/jo0710432. Epub 2007 Jul 27. J Org Chem. 2007. PMID: 17655366 Free PMC article.
-
Facile Access to Cyclooctanoid Ring Systems via Microwave-Assisted Tandem 6-exo dig Cyclization-Rearrangement Sequence.Tetrahedron. 2014 Jul 8;70(27-28):4147-4155. doi: 10.1016/j.tet.2014.02.089. Tetrahedron. 2014. PMID: 24994941 Free PMC article.
-
Tandem anionic 5-Exo dig cyclization/Claisen rearrangement as an efficient route to fused polycyclic ring systems.Org Lett. 2000 Jul 27;2(15):2361-4. doi: 10.1021/ol006139w. Org Lett. 2000. PMID: 10930284
-
[Development of new synthetic reactions featuring tandem carbon-carbon bond formation].Yakugaku Zasshi. 2007 Sep;127(9):1399-418. doi: 10.1248/yakushi.127.1399. Yakugaku Zasshi. 2007. PMID: 17827921 Review. Japanese.
-
5-Endo-dig cyclizations in organic syntheses.Mol Divers. 2020 Feb;24(1):295-317. doi: 10.1007/s11030-019-09930-x. Epub 2019 Mar 5. Mol Divers. 2020. PMID: 30838496 Review.
Cited by
-
Highly Enantioselective Catalytic Lactonization at Nonactivated Primary and Secondary γ-C-H Bonds.J Am Chem Soc. 2023 Aug 16;145(32):18094-18103. doi: 10.1021/jacs.3c06231. Epub 2023 Aug 4. J Am Chem Soc. 2023. PMID: 37540636 Free PMC article.
-
The effect of neighbouring group participation and possible long range remote group participation in O-glycosylation.Beilstein J Org Chem. 2025 Feb 17;21:369-406. doi: 10.3762/bjoc.21.27. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 39996165 Free PMC article. Review.
-
Carboxylic Acid Directed γ-Lactonization of Unactivated Primary C-H Bonds Catalyzed by Mn Complexes: Application to Stereoselective Natural Product Diversification.J Am Chem Soc. 2022 Oct 26;144(42):19542-19558. doi: 10.1021/jacs.2c08620. Epub 2022 Oct 13. J Am Chem Soc. 2022. PMID: 36228322 Free PMC article.
References
-
- Mehta G; Singh V Progress in the Construction of Cyclooctanoid Systems: New Approaches and Applications to Natural Product Syntheses. Chem. Rev 1999, 99, 881–930. - PubMed
- Hu Y-J; Li L-X; Han J-C; Min L; Li C-C Recent Advances in the Total Synthesis of Natural Products Containing Eight-Membered Carbocycles (2009–2019). Chem. Rev 2020, 120, 5910. - PubMed
-
- Petasis NA; Patane MA The synthesis of carbocyclic eight-membered rings. Tetrahedron 1992, 48, 5757–5821.
-
- Paquette LA; Ham WH Total synthesis of the marine sesquiterpenes dactylol and africanol. De novo construction of a cyclooctanoid natural product from cycloheptane precursors. J. Am. Chem. Soc 1987, 109, 3025–3036.
- Birch AM; Pattenden G Total synthesis of epi-precapnelladiene. J. Chem. Soc., Chem. Commun 1980, 1195–1197.
- Mehta G; Murthy AN A General Stereocontrolled Approach to the 5–8 Fused Ring System. Applications to the Total Synthesis of Marine Natural Product (±)-Precapnelladiene. J. Org. Chem 1987, 52, 2875–2881.
- Feldman KS; Wu MJ; Rotella DP Total synthesis of (±)-dactylol and related studies. J. Am. Chem. Soc 1990, 112, 8490–8496.
-
- For a review, see Ojima I; Tzamarioudaki M; Li Z; Donovan RJ Transition Metal-Catalyzed Carbocyclizations in Organic Synthesis. Chem. Rev 1996, 96, 635–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

