Conjugated activation of myocardial-specific transcription of Gja5 by a pair of Nkx2-5-Shox2 co-responsive elements
- PMID: 32687896
- PMCID: PMC7484358
- DOI: 10.1016/j.ydbio.2020.07.003
Conjugated activation of myocardial-specific transcription of Gja5 by a pair of Nkx2-5-Shox2 co-responsive elements
Abstract
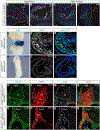
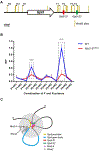
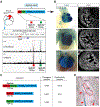
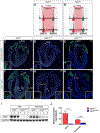
The sinoatrial node (SAN) is the primary pacemaker in the heart. During cardiogenesis, Shox2 and Nkx2-5 are co-expressed in the junction domain of the SAN and regulate pacemaker cell fate through a Shox2-Nkx2-5 antagonism. Cx40 is a marker of working myocardium and an Nkx2-5 transcriptional output antagonized by Shox2, but the underlying regulatory mechanisms remain elusive. Here we characterized a bona fide myocardial-specific Gja5 (coding gene of Cx40) distal enhancer consisting of a pair of Nkx2-5 and Shox2 co-bound elements in the regulatory region of Gja5. Transgenic reporter assays revealed that neither element alone, but the conjugation of both elements together, drives myocardial-specific transcription. Genetic analyses confirmed that the activation of this enhancer depends on Nkx2-5 but is inhibited by Shox2 in vivo, and its presence is essential for Gja5 expression in the myocardium but not the endothelial cells of the heart. Furthermore, chromatin conformation analysis showed an Nkx2-5-dependent loop formation between these two elements and the Gja5 promoter in vivo, indicating that Nkx2-5 bridges the conjugated activation of this enhancer by pairing the two elements to the Gja5 promoter.
Keywords: Enhancer; Gja5; Myocardium; Nkx2-5; Shox2; Sinoatrial node.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing or financial interests.
Figures
References
-
- Ambrosetti DC, Basilico C, Dailey L, 1997. Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct-3 depends on protein-protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol. Cell. Biol 17, 6321–6329. 10.1128/mcb.17.11.6321 - DOI - PMC - PubMed
-
- Anderson CM, Hu J, Thomas R, Gainous TB, Celona B, Sinha T, Dickel DE, Heidt AB, Xu SM, Bruneau BG, Pollard KS, Pennacchio LA, Black BL, 2017. Cooperative activation of cardiac transcription through myocardin bridging of paired MEF2 sites. Development 144, 1235–1241. 10.1242/dev.138487 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

