An updated insight into the molecular pathogenesis, secondary complications and potential therapeutics of COVID-19 pandemic
- PMID: 32687917
- PMCID: PMC7366108
- DOI: 10.1016/j.lfs.2020.118105
An updated insight into the molecular pathogenesis, secondary complications and potential therapeutics of COVID-19 pandemic
Abstract
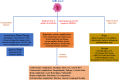
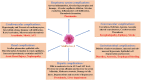
Coronavirus disease 2019 (COVID-19) is an unprecedented disease caused by highly pathogenic SARS-CoV-2 and characterized by extreme respiratory deterrence, pneumonia and immune damage. The phylogenetic analysis demonstrated the sequence similarity of SARS-CoV-2 with other SARS-like bat viruses. The primary source and intermediate host are not yet confirmed, although transmission from human to human is universally confirmed. The new SARS-CoV-2 virus reaches cells via ACE-2 and subsequently down-regulates ACE-2, leaving angiotensin II unbalanced in affected organs primarily in the lungs, heart, brain, and kidneys. As reported recently, numerous secondary complications i.e., neurological, nephrological, cardiovascular, gastrointestinal, immune, and hepatic complications, are associated with COVID-19 infection along with prominent respiratory disease including pneumonia. Extensive research work on recently discovered SARS-CoV-2 is in the pipeline to clarify pathogenic mechanisms, epidemiological features, and identify new drug targets that will lead to the development of successful strategies for prevention and treatment. There are currently no appropriate scientifically approved vaccines/drugs for COVID-19. Nonetheless, few broad-spectrum antiviral drugs, azithromycin were tested against COVID-19 in clinical trials, and finally, FDA approved emergency use of remdesivir in hospitalized COVID-19 patients. Additionally, administration of convalescent plasma obtained from recovered COVID-19 patients to infected COVID-19 patients reduces the viral burden via immunomodulation. This review analysis therefore concentrates primarily on recent discoveries related to COVID-19 pathogenesis along with a full description of the structure, genome, and secondary complication associated with SARS-CoV-2. Finally, a short and brief clinical update has been provided concerning the development of therapeutic medications and vaccines to counter COVID-19.
Keywords: Anti-viral drugs; COVID-19; Convalescent plasma; SARS-CoV-2; Vaccines.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures



References
-
- Hassan S.A., Sheikh F.N., Jamal S., Ezeh J.K., Akhtar A. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus. 2020;12 doi: 10.7759/cureus.7355. https://doi - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

