Investigation of Dalea parryi (Fabaceae) metabolites for anthelmintic activity against the human pathogenic hookworm Ancylostoma ceylanicum
- PMID: 32688268
- PMCID: PMC10798583
- DOI: 10.1016/j.phytochem.2020.112423
Investigation of Dalea parryi (Fabaceae) metabolites for anthelmintic activity against the human pathogenic hookworm Ancylostoma ceylanicum
Abstract
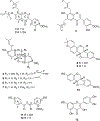
The US Southwest plant Dalea parryi (Fabaceae) was investigated as part of an ongoing study of the potential of plant compounds for anthelmintic activity to the human pathogenic hookworm Ancylostoma ceylanicum. This has resulted in the isolation of three previously undescribed isoflavonoid metabolites, denoted parryans A-C, a chalcone, six pterocarpans, and three known compounds from the roots of D. parryi. Parryans A and B express a rarely-seen O-prenyl substituent. Structures of the previously undescribed compounds were established using 1D and 2D NMR spectroscopy and mass spectrometry. The relative and absolute configurations of the undescribed stereoisomers were assigned using chemical shift and coupling constant data and comparisons of specific rotations to published data. The most active of the isolated compounds only expressed a 17% reduction in survival of A. ceylanicum adult hookworm in an ex vivo assay at 50 μg/mL after 5 days exposure. Toxicity, ranging from 47 to 93% reduction in survival of mammalian splenocytes was expressed by four of the compounds.
Keywords: Ancylostoma ceylanicum; Anthelmintic activity; Chalcone; Dalea parryi; Fabaceae; Isoflavone; Pterocarpan; Toxicity.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest
No potential conflict of interest was reported by the authors.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Asada Y, Li W, Yoshikawa T 1998. Isoprenylated flavonoids from hairy root cultures of Glycyrrhiza glabra. Phytochemistry 47, 389–392.
-
- Ayabe S-I, Kobayashi M, Hikichi M, Matsumoto K, Furuya T 1980. Flavonoids from the cultured cells of Glycyrrhiza echinata. Phytochemistry 19, 2179–2183.
-
- Baskin JM, Baskin CC 1985. Photosynthetic pathway in 14 Southeastern cedar glade endemics, as revealed by leaf anatomy. Am. Midl. Nat 114, 205–208.
-
- Belofsky G, Carreno R, Lewis K, Ball A, Casadei G, Tegos GP 2006. Metabolites of the “Smoke Tree”, Dalea spinosa, Potentiate Antibiotic Activity against Multi-Drug Resistant Staphylococcus aureus. J. Nat. Prod 69, 261–264. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources