Are supplemental appraisal/reimbursement processes needed for rare disease treatments? An international comparison of country approaches
- PMID: 32690107
- PMCID: PMC7370450
- DOI: 10.1186/s13023-020-01462-0
Are supplemental appraisal/reimbursement processes needed for rare disease treatments? An international comparison of country approaches
Abstract
Background: There is increasing recognition that conventional appraisal approaches may be unsuitable for assessing the value rare disease treatments (RDTs). This research examines what supplemental appraisal/reimbursement processes for RDTs are used internationally and how they can be characterised. A qualitative research design was used that included (1) documentation of country appraisal/reimbursement processes for RDTs via questionnaires, desk research and iterative interactions with country experts to produce country vignettes, and (2) a cross-country analysis of these processes to identify and characterise features in supplemental processes for RDTs, and compare them to countries without supplemental processes.
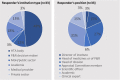
Results: Thirty-two of the 37 invited countries participated in this research. Forty-one percent (13/32) use supplemental processes for RDTs. Their level of integration within standard processes ranged from low to high, characterised by whether they are separate or partially separate from the standard process, adapted or accelerated standard processes, or standard processes that may be applied to RDTs. They are characterised by features implemented throughout the appraisal process. These features are mechanisms that allow application of different standards to assess the value of the medicine, support to the appraisal/decision-making process, overcome the issues of lack of cost-effectiveness, or exempt from part of/the full appraisal/reimbursement process. They increase the likelihood of reimbursement by adjusting and/or foregoing part of the assessment process, or accepting to pay more for the same added benefit as for common conditions. A large proportion of countries with standard processes include one or more of these features (formally or informally) or are discussing potential changes in their systems.
Conclusions: Results suggest revealed preferences to treat RDTs differently than conventional medicines. Some of the challenges around uncertainty and high price remain, but supplemental process features can support decision-making that is more flexible and consistent. Many of these processes are new and countries continue to adjust as they gain experience.
Keywords: Access to treatments; Appraisal; Health technology assessment; Orphan medicine; Rare disease treatment; Reimbursement; Supplemental processes; Ultra-orphan medicine.
Conflict of interest statement
EN is part time employed and AW is contracted on a project base by Dolon Ltd. They do not have any competing interests with this work. MD and KF do not have any conflict of interests to declare.
Figures


References
-
- European Commission. Orphan medicinal products. [Cited 2020 Jan 20]. Available from: https://ec.europa.eu/health/human-use/orphan-medicines_en.
-
- UK Genetic Alliance . Action for access: a report from genetic Alliance UK for the all party parliamentary group on rare, genetic and undiagnosed conditions. 2019.
-
- Nestler-Parr S, Korchagina D, Toumi M, Pashos CL, Blanchette C, Molsen E, et al. Challenges in research and health technology assessment of rare disease technologies: report of the ISPOR rare disease special interest group. Value Health. 2018;21:493–500. doi: 10.1016/j.jval.2018.03.004. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

