Tracking cells in epithelial acini by light sheet microscopy reveals proximity effects in breast cancer initiation
- PMID: 32690136
- PMCID: PMC7373425
- DOI: 10.7554/eLife.54066
Tracking cells in epithelial acini by light sheet microscopy reveals proximity effects in breast cancer initiation
Abstract
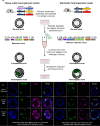
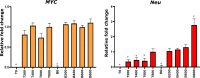
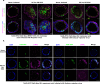
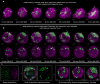
Cancer clone evolution takes place within tissue ecosystem habitats. But, how exactly tumors arise from a few malignant cells within an intact epithelium is a central, yet unanswered question. This is mainly due to the inaccessibility of this process to longitudinal imaging together with a lack of systems that model the progression of a fraction of transformed cells within a tissue. Here, we developed a new methodology based on primary mouse mammary epithelial acini, where oncogenes can be switched on in single cells within an otherwise normal epithelial cell layer. We combine this stochastic breast tumor induction model with inverted light-sheet imaging to study single-cell behavior for up to four days and analyze cell fates utilizing a newly developed image-data analysis workflow. The power of this integrated approach is illustrated by us finding that small local clusters of transformed cells form tumors while isolated transformed cells do not.
Keywords: cancer biology; cell biology; interaction requirements for tumor initiation; light sheet imaging technology; mouse; organoid technology of primary mammary epithelium; scalable big image data analysis pipeline; tractable tumor induction system.
Plain language summary
There are now drugs to treat many types of cancer, but questions still remain around how these diseases start in the first place. Researchers think that tumor growth begins when a single cell suffers damage to certain sites in its DNA that eventually cause it to divide uncontrollably. That damaged cell, and its descendants, go on to form a lump, or tumor. The trouble with proving this theory is that it is hard to watch it happening in real time. Doctors usually only meet people with cancer when their tumors start to cause health problems. By this point, the tumors contain millions of cells. A way to watch the very beginnings of a cancer could reveal risk factors within a tissue that foster the growth of a tumor. But first, researchers need to test their theory about how the disease begins in the first place. One way to do this is to surround a single cancer cell with healthy cells and watch what happens next. To do this, Alladin, Chaible et al. took healthy cells from the breast tissue of mice and grew them in the laboratory into mini-organs called organoids. These organoids share a lot of features with actual mouse breast tissue; they can even make milk if given the right hormones. Once the organoids were ready, Alladin, Chaible et al then started modifying a small number of single cells inside them by switching on genes called oncogenes, which are known to drive cancer formation in humans. Using fluorescent proteins and a sheet of laser light it was possible to watch what happened to the cells over time. This revealed that, even though all the oncogene-driven single cells received the same signals, not all of them started to divide uncontrollably. In fact, a single modified cell had a low chance of forming a tumor on its own. The more oncogene-driven cells there were near to each other, the more likely they were to form tumors. Alladin, Chaible et al. think that this is because the healthy tissue interacts with the modified, oncogene-driven cells to suppress tumor formation. It is only when a larger number of modified cells group together and start to communicate with each other that they can override the inhibitory messages of the healthy tissue. How healthy tissue stops single modified cells from forming tumors is not yet clear. But, with this new mini-organ system, researchers now have the tools to investigate. In the future, this could lead to new strategies to stop cancer before it has a chance to get started.
© 2020, Alladin et al.
Conflict of interest statement
AA, LC, LG, RS, JH, CT, MJ No competing interests declared, ML Monika Loeschinger is employed by Luxendo GmbH, FM BU, Bruker Nano Surfaces, Heidelberg, Germany, the manufacturer of the InVi SPIM light-sheet microscope, MW Malte Wachsmuth is employed by Luxendo GmbH, FM BU, Bruker Nano Surfaces, Heidelberg, Germany, the manufacturer of the InVi SPIM light-sheet microscope
Figures


Similar articles
-
Induction of cells with cancer stem cell properties from nontumorigenic human mammary epithelial cells by defined reprogramming factors.Oncogene. 2014 Jan 30;33(5):643-52. doi: 10.1038/onc.2012.614. Epub 2013 Jan 14. Oncogene. 2014. PMID: 23318426 Free PMC article.
-
Mammary epithelial cell transformation: insights from cell culture and mouse models.Breast Cancer Res. 2005;7(4):171-9. doi: 10.1186/bcr1275. Epub 2005 Jun 3. Breast Cancer Res. 2005. PMID: 15987472 Free PMC article. Review.
-
EMT inducers catalyze malignant transformation of mammary epithelial cells and drive tumorigenesis towards claudin-low tumors in transgenic mice.PLoS Genet. 2012;8(5):e1002723. doi: 10.1371/journal.pgen.1002723. Epub 2012 May 24. PLoS Genet. 2012. PMID: 22654675 Free PMC article.
-
Functional purification of human and mouse mammary stem cells.Methods Mol Biol. 2012;916:59-79. doi: 10.1007/978-1-61779-980-8_6. Methods Mol Biol. 2012. PMID: 22914933
-
Role of epithelial stem/progenitor cells in mammary cancer.Gene Expr. 2011;15(3):133-40. doi: 10.3727/105221611x13176664479368. Gene Expr. 2011. PMID: 22268295 Free PMC article. Review.
Cited by
-
Beyond genetics: driving cancer with the tumour microenvironment behind the wheel.Nat Rev Cancer. 2024 Apr;24(4):274-286. doi: 10.1038/s41568-023-00660-9. Epub 2024 Feb 12. Nat Rev Cancer. 2024. PMID: 38347101 Free PMC article. Review.
-
Quantifying the relationship between cell proliferation and morphology during development of the face.Development. 2025 Apr 1;152(7):dev204511. doi: 10.1242/dev.204511. Epub 2025 Apr 7. Development. 2025. PMID: 39989423 Free PMC article.
-
Boosting the Anticancer Activity of Sunitinib Malate in Breast Cancer through Lipid Polymer Hybrid Nanoparticles Approach.Polymers (Basel). 2022 Jun 16;14(12):2459. doi: 10.3390/polym14122459. Polymers (Basel). 2022. PMID: 35746034 Free PMC article.
-
Light and electron microscopy continuum-resolution imaging of 3D cell cultures.Dev Cell. 2023 Apr 10;58(7):616-632.e6. doi: 10.1016/j.devcel.2023.03.001. Epub 2023 Mar 28. Dev Cell. 2023. PMID: 36990090 Free PMC article.
-
Current and future applications of light-sheet imaging for identifying molecular and developmental processes in autism spectrum disorders.Mol Psychiatry. 2024 Jul;29(7):2274-2284. doi: 10.1038/s41380-024-02487-8. Epub 2024 Mar 5. Mol Psychiatry. 2024. PMID: 38443634 Review.
References
-
- Al-Kuraya K, Schraml P, Torhorst J, Tapia C, Zaharieva B, Novotny H, Spichtin H, Maurer R, Mirlacher M, Köchli O, Zuber M, Dieterich H, Mross F, Wilber K, Simon R, Sauter G. Prognostic relevance of gene amplifications and coamplifications in breast Cancer. Cancer Research. 2004;64:8534–8540. doi: 10.1158/0008-5472.CAN-04-1945. - DOI - PubMed
-
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. - DOI - PMC - PubMed
-
- Bitplane AG 3/4D Image Visualization and Analysis Software. v. x64Imaris. 2020 http://bitplane.com
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

