Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: A retrospective cohort study
- PMID: 32690352
- PMCID: PMC7365106
- DOI: 10.1016/j.jaut.2020.102523
Experience with tocilizumab in severe COVID-19 pneumonia after 80 days of follow-up: A retrospective cohort study
Abstract
Objectives: To describe the clinical characteristics and predictors of major outcomes in patients treated with tocilizumab (TCZ) for severe COVID-19 pneumonia.
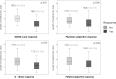
Patients and methods: Case series of all sequential patients with severe COVID-19 pneumonia treated with TCZ at an Academic Spanish hospital (March 12 - May 2, 2020). Clinical outcomes: death, length of hospital stay. An early clinical response to TCZ (48-72 h after the administration) was assessed by variations in respiratory function markers, Brescia COVID Respiratory Severity Scale (BCRSS), inflammatory parameters, and patients' and physicians' opinion. Associations were tested by multiple logistic regression.
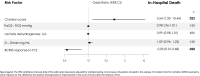
Results: From a cohort of 236 patients, 77 patients treated with TCZ were included (median age 62 years (IQR 53.0-72.0), 64.9% were males), 42.9% had Charlson index ≥3; hypertension (41.6%), obesity (34.7%), and diabetes (20.8%). Median follow-up was 83.0 days (78.0-86.5), no patient was readmitted. ICU admission was required for 42 (54.5%), invasive mechanical ventilation in 38 (49.4%) and 10 patients died (12.9% global, 23.8% at ICU admitted). After multivariate adjustment, TCZ response by BCRSS (OR 0.03 (0.01-0.68), p = 0.028), and Charlson index (OR 3.54 (1.20-10.44), p = 0.022) has been identified as independent factors associated with mortality. Median of hospital stay was 16.0 days (11.0-23.0); BCRSS, physician subjective and D-dimer response were associated with shorter hospitalization stay.
Conclusions: In a Mediterranean cohort, use of tocilizumab for severe COVID-19 show 12.9% of mortality. Early TCZ-response by BCRSS and low comorbidity were associated with increased survival. Early TCZ-response was related to shorter median hospital stay.
Keywords: COVID19 pneumonia; Case series; Mechanical invasive ventilation; Mortality; Tocilizumab.
Published by Elsevier Ltd.
Conflict of interest statement
MA declares speaking fees from Roche Pharma (<10,000$).
Figures
References
-
- Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C.-C., Edwards K.M., Gandhi R., Muller W.J., O'Horo J.C., Shoham S., Murad M.H., Mustafa R.A., Sultan S., Falck-Ytter Y. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa478. - DOI - PMC - PubMed
-
- Razonable R.R., Pennington K.M., Meehan A.M., Wilson J.W., Froemming A.T., Bennett C.E., Marshall A.L., Virk A., Carmona E.M. A collaborative multidisciplinary approach to the management of coronavirus disease 2019 in the hospital setting. Mayo Clin. Proc. 2020;95:1467–1481. doi: 10.1016/j.mayocp.2020.05.010. - DOI - PMC - PubMed
-
- Duca A., Piva S., Focà E., Latronico N., Rizzi M. Calculated decisions: brescia-COVID respiratory severity scale (BCRSS)/Algorithm. Emerg. Med. Pract. 2020;22:CD1–CD2. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

