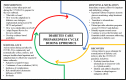
Preparedness cycle to address transitions in diabetes care during the COVID-19 pandemic and future outbreaks
- PMID: 32690631
- PMCID: PMC7385737
- DOI: 10.1136/bmjdrc-2020-001520
Preparedness cycle to address transitions in diabetes care during the COVID-19 pandemic and future outbreaks
Abstract
The COVID-19 pandemic is considered a mass casualty incident of the most severe nature leading to unearthed uncertainties around management, prevention, and care. As of July 2020, more than twelve million people have tested positive for COVID-19 globally and more than 500 000 people have died. Patients with diabetes are among the most severely affected during this pandemic. Healthcare systems have made emergent changes to adapt to this public health crisis, including changes in diabetes care. Adaptations in diabetes care in the hospital (ie, changes in treatment protocols according to clinical status, diabetes technology implementation) and outpatient setting (telemedicine, mail delivery, patient education, risk stratification, monitoring) have been improvised to address this challenge. We describe how to respond to the current public health crisis focused on diabetes care in the USA. We present strategies to address and evaluate transitions in diabetes care occurring in the immediate short-term (ie, response and mitigation), as well as phases to adapt and enhance diabetes care during the months and years to come while also preparing for future pandemics (ie, recovery, surveillance, and preparedness). Implementing multidimensional frameworks may help identify gaps in care, alleviate initial demands, mitigate potential harms, and improve implementation strategies and outcomes in the future.
Keywords: delivery of health care; diabetes complications; diabetes mellitus, type 2.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PV has received consulting fees from Merck and Boehringer Ingelheim. FJP has received unrestricted research support from Merck and Dexcom and consulting fees from Merck, Boehringer Ingelheim, Sanofi, Lilly, and AstraZeneca.
Figures
References
-
- COVID-19 Map Johns Hopkins coronavirus resource center. Available: https://coronavirus.jhu.edu/map.html [Accessed 10 July 2020].
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020;323:1239–42. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials