Combination of Miconazole and Domiphen Bromide Is Fungicidal against Biofilms of Resistant Candida spp
- PMID: 32690639
- PMCID: PMC7508569
- DOI: 10.1128/AAC.01296-20
Combination of Miconazole and Domiphen Bromide Is Fungicidal against Biofilms of Resistant Candida spp
Abstract
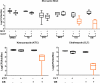
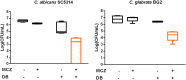
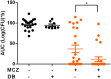
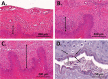
The occurrence and recurrence of mucosal biofilm-related Candida infections, such as oral and vulvovaginal candidiasis, are serious clinical issues. Vaginal infections caused by Candida spp., for example, affect 70 to 75% of women at least once during their lives. Miconazole (MCZ) is the preferred topical treatment against these fungal infections, yet it has only moderate antibiofilm activity. Through screening of a drug-repurposing library, we identified the quaternary ammonium compound domiphen bromide (DB) as an MCZ potentiator against Candida biofilms. DB displayed synergistic anti-Candida albicans biofilm activity with MCZ, reducing the number of viable biofilm cells 1,000-fold. In addition, the MCZ-DB combination also resulted in significant killing of biofilm cells of azole-resistant C. albicans, C. glabrata, and C. auris isolates. In vivo, the MCZ-DB combination had significantly improved activity in a vulvovaginal candidiasis rat model compared to that of single-compound treatments. Data from an artificial evolution experiment indicated that the development of resistance against the combination did not occur, highlighting the potential of MCZ-DB combination therapy to treat Candida biofilm-related infections.
Keywords: Candida; biofilms; combination treatment; domiphen bromide; drug repurposing; fungicidal; miconazole; resistance development; vulvovaginal candidiasis.
Copyright © 2020 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

