Rifampicin Transport by OATP1B1 Variants
- PMID: 32690641
- PMCID: PMC7508598
- DOI: 10.1128/AAC.00955-20
Rifampicin Transport by OATP1B1 Variants
Abstract
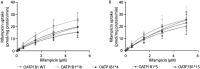
Single nucleotide polymorphisms in the OATP1B1 transporter have been suggested to partially explain the large interindividual variation in rifampicin exposure. HEK293 cells overexpressing wild-type (WT) or OATP1B1 variants *1b, *4, *5, and *15 were used to determine the in vitro rifampicin intrinsic clearance. For OATP1B1*5 and *15, a 36% and 42% reduction in intrinsic clearance, respectively, compared to WT was found. We consider that these differences in intrinsic clearance most likely have minor clinical implications.
Keywords: OATP1B1; SNP; drug transport; rifampicin.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- WHO. 2019. Global tuberculosis report. World Health Organization, Geneva, Switzerland: License: CC BY-NC-SA 3.0 IGO.
-
- Stott KE, Pertinez H, Sturkenboom MGG, Boeree MJ, Aarnoutse R, Ramachandran G, Requena-Mendez A, Peloquin C, Koegelenberg CFN, Alffenaar JWC, Ruslami R, Tostmann A, Swaminathan S, McIlleron H, Davies G. 2018. Pharmacokinetics of rifampicin in adult TB patients and healthy volunteers: a systematic review and meta-analysis. J Antimicrob Chemother 73:2305–2313. doi:10.1093/jac/dky152. - DOI - PMC - PubMed
-
- Weiner M, Benator D, Burman W, Peloquin CA, Khan A, Vernon A, Jones B, Silva-Trigo C, Zhao Z, Hodge T, Tuberculosis Trials Consortium. 2005. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis 40:1481–1491. doi:10.1086/429321. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

