Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress
- PMID: 32690710
- PMCID: PMC7414143
- DOI: 10.1073/pnas.2009425117
Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress
Abstract
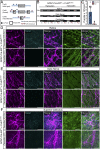
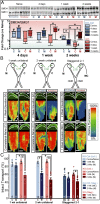
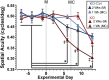
In the central nervous system, glycogen-derived bioenergetic resources in astrocytes help promote tissue survival in response to focal neuronal stress. However, our understanding of the extent to which these resources are mobilized and utilized during neurodegeneration, especially in nearby regions that are not actively degenerating, remains incomplete. Here we modeled neurodegeneration in glaucoma, the world's leading cause of irreversible blindness, and measured how metabolites mobilize through astrocyte gap junctions composed of connexin 43 (Cx43). We elevated intraocular pressure in one eye and determined how astrocyte-derived metabolites in the contralateral optic projection responded. Remarkably, astrocyte networks expand and redistribute metabolites along distances even 10 mm in length, donating resources from the unstressed to the stressed projection in response to intraocular pressure elevation. While resource donation improves axon function and visual acuity in the directly stressed region, it renders the donating tissue susceptible to bioenergetic, structural, and physiological degradation. Intriguingly, when both projections are stressed in a WT animal, axon function and visual acuity equilibrate between the two projections even when each projection is stressed for a different length of time. This equilibration does not occur when Cx43 is not present. Thus, Cx43-mediated astrocyte metabolic networks serve as an endogenous mechanism used to mitigate bioenergetic stress and distribute the impact of neurodegenerative disease processes. Redistribution ultimately renders the donating optic nerve vulnerable to further metabolic stress, which could explain why local neurodegeneration does not remain confined, but eventually impacts healthy regions of the brain more broadly.
Keywords: astrocyte; astrocyte network; gap junction; metabolism; neurodegeneration.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Astrocyte Networks as Therapeutic Targets in Glaucomatous Neurodegeneration.Cells. 2021 Jun 2;10(6):1368. doi: 10.3390/cells10061368. Cells. 2021. PMID: 34199470 Free PMC article. Review.
-
Adaptive responses to neurodegenerative stress in glaucoma.Prog Retin Eye Res. 2021 Sep;84:100953. doi: 10.1016/j.preteyeres.2021.100953. Epub 2021 Feb 25. Prog Retin Eye Res. 2021. PMID: 33640464 Free PMC article. Review.
-
Pressure induces loss of gap junction communication and redistribution of connexin 43 in astrocytes.Glia. 2007 Aug 1;55(10):1085-98. doi: 10.1002/glia.20527. Glia. 2007. PMID: 17551925
-
Megalencephalic Leukoencephalopathy with Subcortical Cysts Disease-Linked MLC1 Protein Favors Gap-Junction Intercellular Communication by Regulating Connexin 43 Trafficking in Astrocytes.Cells. 2020 Jun 8;9(6):1425. doi: 10.3390/cells9061425. Cells. 2020. PMID: 32521795 Free PMC article.
-
Astrocyte remodeling without gliosis precedes optic nerve Axonopathy.Acta Neuropathol Commun. 2018 May 10;6(1):38. doi: 10.1186/s40478-018-0542-0. Acta Neuropathol Commun. 2018. PMID: 29747701 Free PMC article.
Cited by
-
Engineering a 3D hydrogel system to study optic nerve head astrocyte morphology and behavior.Exp Eye Res. 2022 Jul;220:109102. doi: 10.1016/j.exer.2022.109102. Epub 2022 May 5. Exp Eye Res. 2022. PMID: 35525298 Free PMC article.
-
Multifactorial Pathogenic Processes of Retinal Ganglion Cell Degeneration in Glaucoma towards Multi-Target Strategies for Broader Treatment Effects.Cells. 2021 Jun 2;10(6):1372. doi: 10.3390/cells10061372. Cells. 2021. PMID: 34199494 Free PMC article. Review.
-
Astrocytes in selective vulnerability to neurodegenerative disease.Trends Neurosci. 2024 Apr;47(4):289-302. doi: 10.1016/j.tins.2024.02.008. Epub 2024 Mar 22. Trends Neurosci. 2024. PMID: 38521710 Free PMC article. Review.
-
In vivo spatiotemporal dynamics of astrocyte reactivity following neural electrode implantation.Biomaterials. 2022 Oct;289:121784. doi: 10.1016/j.biomaterials.2022.121784. Epub 2022 Sep 2. Biomaterials. 2022. PMID: 36103781 Free PMC article.
-
Restoring the Extracellular Matrix: A Neuroprotective Role for Collagen Mimetic Peptides in Experimental Glaucoma.Front Pharmacol. 2021 Nov 2;12:764709. doi: 10.3389/fphar.2021.764709. eCollection 2021. Front Pharmacol. 2021. PMID: 34795592 Free PMC article.
References
-
- Duarte J. M., Girault F. M., Gruetter R., Brain energy metabolism measured by (13)C magnetic resonance spectroscopy in vivo upon infusion of [3-(13)C]lactate. J. Neurosci. Res. 93, 1009–1018 (2015). - PubMed
-
- Brown A. M. et al. ., Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J. Neurosci. Res. 79, 74–80 (2005). - PubMed
-
- Swanson R. A., Morton M. M., Sagar S. M., Sharp F. R., Sensory stimulation induces local cerebral glycogenolysis: Demonstration by autoradiography. Neuroscience 51, 451–461 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

