Evidence-based prevention of Alzheimer's disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials
- PMID: 32690803
- PMCID: PMC7569385
- DOI: 10.1136/jnnp-2019-321913
Evidence-based prevention of Alzheimer's disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials
Abstract
Background: Evidence on preventing Alzheimer's disease (AD) is challenging to interpret due to varying study designs with heterogeneous endpoints and credibility. We completed a systematic review and meta-analysis of current evidence with prospective designs to propose evidence-based suggestions on AD prevention.
Methods: Electronic databases and relevant websites were searched from inception to 1 March 2019. Both observational prospective studies (OPSs) and randomised controlled trials (RCTs) were included. The multivariable-adjusted effect estimates were pooled by random-effects models, with credibility assessment according to its risk of bias, inconsistency and imprecision. Levels of evidence and classes of suggestions were summarised.
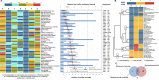
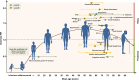
Results: A total of 44 676 reports were identified, and 243 OPSs and 153 RCTs were eligible for analysis after exclusion based on pre-decided criteria, from which 104 modifiable factors and 11 interventions were included in the meta-analyses. Twenty-one suggestions are proposed based on the consolidated evidence, with Class I suggestions targeting 19 factors: 10 with Level A strong evidence (education, cognitive activity, high body mass index in latelife, hyperhomocysteinaemia, depression, stress, diabetes, head trauma, hypertension in midlife and orthostatic hypotension) and 9 with Level B weaker evidence (obesity in midlife, weight loss in late life, physical exercise, smoking, sleep, cerebrovascular disease, frailty, atrial fibrillation and vitamin C). In contrast, two interventions are not recommended: oestrogen replacement therapy (Level A2) and acetylcholinesterase inhibitors (Level B).
Interpretation: Evidence-based suggestions are proposed, offering clinicians and stakeholders current guidance for the prevention of AD.
Keywords: alzheimer's disease; epidemiology; meta-analysis; systematic reviews.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JTY serves as an associate editor-in-chief for Annals of Translational Medicineand is senior editor for Journal of Alzheimer’s Disease. SA has received grants from Europe, Ipsen, and France Alzheimer, served as a consultant for Ipsen, Pierre Fabre, Lilly, Nestlé, Sanofi and Servier, and received non-financial support from Biogen, Nutrition Santé, Pfzer and Icon, and other support from the AMPA Association. GS has received clinical trial support from Lilly and Roche in DIAN-TU, TauRx Therapeutics (TauRx) and Lundbeck; has been a data safety monitoring board (DSMB) member of ADCS, ATRI, API and Eisai; and has been a scientific adviser to Affiris, Boehringer Ingelheim, Lilly, Roche, Servier, Sanofi, Schwabe, Takeda and TauRx. PSA has received grants from the US Alzheimer’s Association, Janssen, Lilly, the US National Institute on Aging and Toyama; and consulting fees from Abbott, Abbvie, Amgen, Anavex, AstraZeneca, Biogen Idec, Biotie, Bristol-Myers Squibb, Cardeus, Cohbar, Eisai, Elan, Eli Lilly, Genentech, Ichor, iPerian, Janssen, Lundbeck, Medivation, Merck, NeuroPhage, Novartis, Pfizer, Probiodrug, Roche, Somaxon and Toyama, outside the submitted work. BV reports grants from Pierre Fabre, Avid, Exonhit, AbbVie, Lilly, Lundbeck, MSD, Otsuka, Regenron, Sanofi, Roche, AstraZeneca, LPG Systems, Nestlé and Alzheon, and personal fees from Lilly, Lundbeck, MSD, Otsuka, Roche, Sanofi, Biogen, Nestlé, Transition Therapeutics and Takeda.
Figures



Comment in
-
Prevention of Alzheimer's disease and dementia: the evidence is out there, but new high-quality studies and implementation are needed.J Neurol Neurosurg Psychiatry. 2020 Nov;91(11):1140-1141. doi: 10.1136/jnnp-2020-323606. Epub 2020 Sep 15. J Neurol Neurosurg Psychiatry. 2020. PMID: 32934001 No abstract available.
References
-
- Matthews FE, Arthur A, Barnes LE, et al. . A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382:1405–12. 10.1016/S0140-6736(13)61570-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
