TREC and KREC profiling as a representative of thymus and bone marrow output in patients with various inborn errors of immunity
- PMID: 32691468
- PMCID: PMC7488118
- DOI: 10.1111/cei.13484
TREC and KREC profiling as a representative of thymus and bone marrow output in patients with various inborn errors of immunity
Abstract
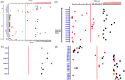
Primary immune deficiency (PID) disorders are clinically and molecularly heterogeneous diseases. T cell receptor excision circles (TRECs) and κ (kappa)-deleting excision circles (KRECs) are markers of T and B cell development, respectively. They are useful tools to assess T and B cell function and immune reconstitution and have been used for newborn screening for severe combined immunodeficiency disease (SCID) and agammaglobulinemia, respectively. Their profiles in several genetically confirmed PIDs are still lacking. The objective of this study was to determine TREC and KREC genomic profiling among various molecularly confirmed PIDs. We used real-time-quantitative polymerase chain reaction (RT-qPCR)-based triplex analysis of TRECs, KRECs and β-actin (ACTB) in whole blood genomic DNA isolated from 108 patients with molecularly confirmed PIDs. All agammaglobulinemia patients had low KREC counts. All SCIDs and Omenn syndrome patients secondary to mutations in RAG1, RAG2, DCLRE1C and NHEJ1 had low TREC and KREC counts. JAK3-deficient patients had normal KREC and the TREC count was influenced by the type of mutation. Early-onset ADA patients had low TREC and KREC counts. Four patients with zeta-chain-associated protein kinase 70 (ZAP70) had low TREC. All purine nucleoside phosphorylase (PNP) patients had low TREC. Combined immunodeficiency (CID) patients secondary to AK2, PTPRC, CD247, DCLREC1 and STAT1 had normal TREC and KREC counts. Most patients with ataxia-telangiectasia (AT) patients had low TREC and KREC, while most DOCK8-deficient patients had low TRECs only. Two of five patients with Wiskott-Aldrich syndrome (WAS) had low TREC counts as well as one patient each with bare lymphocyte syndrome (BLS) and chronic granulomatous disease. All patients with Griscelli disease, Chediak-Higashi syndrome, hyper-immunoglobulin (Ig)M syndrome and IFNGR2 had normal TREC and KREC counts. These data suggest that, in addition to classical SCID and agammaglobulinemia, TREC/KREC assay may identify ZAP70 patients and secondary target PIDs, including dedicator of cytokinesis 8 (DOCK8) deficiency, AT and some individuals with WAS and BLS.
Keywords: Hyper IgE; agammaglobulinemia; ataxia telangiectasia; chronic granulomatous disease; severe combined immunodeficiency.
© 2020 British Society for Immunology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

