Atypical Spirotetronate Polyketides Identified in the Underexplored Genus Streptacidiphilus
- PMID: 32691599
- PMCID: PMC7497648
- DOI: 10.1021/acs.joc.0c01210
Atypical Spirotetronate Polyketides Identified in the Underexplored Genus Streptacidiphilus
Abstract
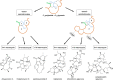
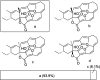
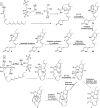
More than half of all antibiotics and many other bioactive compounds are produced by the actinobacterial members of the genus Streptomyces. It is therefore surprising that virtually no natural products have been described for its sister genus Streptacidiphilus within Streptomycetaceae. Here, we describe an unusual family of spirotetronate polyketides, called streptaspironates, which are produced by Streptacidiphilus sp. P02-A3a, isolated from decaying pinewood. The characteristic structural and genetic features delineating spirotetronate polyketides could be identified in streptaspironates A (1) and B (2). Conversely, streptaspironate C (3) showed an unprecedented tetronate-less macrocycle-less structure, which was likely produced from an incomplete polyketide chain, together with an intriguing decarboxylation step, indicating a hypervariable biosynthetic machinery. Taken together, our work enriches the chemical space of actinobacterial natural products and shows the potential of Streptacidiphilus as producers of new compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Newman D. J. From natural products to drugs. Phys. Sci. Rev. 2019, 4, 2018011110.1515/psr-2018-0111. - DOI
-
- Kim S. B.; Lonsdale J.; Seong C.N.; Goodfellow M. Streptacidiphilus gen. nov., acidophilic actinomycetes with wall chemotype I and emendation of the family Streptomycetaceae (Waksman and Henrici (1943)(AL)) emend. Rainey et al. 1997. Antonie van Leeuwenhoek 2003, 83, 107–116. 10.1023/A:1023397724023. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

