The contribution of the mouse tail to thermoregulation is modest
- PMID: 32691633
- PMCID: PMC7473913
- DOI: 10.1152/ajpendo.00133.2020
The contribution of the mouse tail to thermoregulation is modest
Abstract
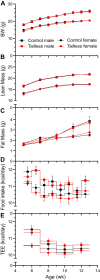
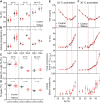
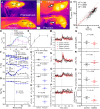
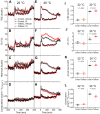
Understanding mouse thermal physiology informs the usefulness of mice as models of human disease. It is widely assumed that the mouse tail contributes greatly to heat loss (as it does in rat), but this has not been quantitated. We studied C57BL/6J mice after tail amputation. Tailless mice housed at 22°C did not differ from littermate controls in body weight, lean or fat content, or energy expenditure. With acute changes in ambient temperature from 19 to 39°C, tailless and control mice demonstrated similar body temperatures (Tb), metabolic rates, and heat conductances and no difference in thermoneutral point. Treatment with prazosin, an α1-adrenergic antagonist and vasodilator, increased tail temperature in control mice by up to 4.8 ± 0.8°C. Comparing prazosin treatment in tailless and control mice suggested that the tail's contribution to total heat loss was a nonsignificant 3.4%. Major heat stress produced by treatment at 30°C with CL316243, a β3-adrenergic agonist, increased metabolic rate and Tb and, at a matched increase in metabolic rate, the tailless mice showed a 0.72 ± 0.14°C greater Tb increase and 7.6% lower whole body heat conductance. Thus, the mouse tail is a useful biomarker of vasodilation and thermoregulation, but in our experiments contributes only 5-8% of whole body heat dissipation, less than the 17% reported for rat. Heat dissipation through the tail is important under extreme scenarios such as pharmacological activation of brown adipose tissue; however, non-tail contributions to heat loss may have been underestimated in the mouse.
Keywords: ambient temperature; body temperature; energy expenditure; heat conductance; tail.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

