Rational design of small molecule fluorescent probes for biological applications
- PMID: 32691820
- PMCID: PMC7453994
- DOI: 10.1039/d0ob01131b
Rational design of small molecule fluorescent probes for biological applications
Abstract
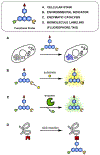
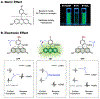
Fluorescent small molecules are powerful tools for visualizing biological events, embodying an essential facet of chemical biology. Since the discovery of the first organic fluorophore, quinine, in 1845, both synthetic and theoretical efforts have endeavored to "modulate" fluorescent compounds. An advantage of synthetic dyes is the ability to employ modern organic chemistry strategies to tailor chemical structures and thereby rationally tune photophysical properties and functionality of the fluorophore. This review explores general factors affecting fluorophore excitation and emission spectra, molar absorption, Stokes shift, and quantum efficiency; and provides guidelines for chemist to create novel probes. Structure-property relationships concerning the substituents are discussed in detail with examples for several dye families. We also present a survey of functional probes based on PeT, FRET, and environmental or photo-sensitivity, focusing on representative recent work in each category. We believe that a full understanding of dyes with diverse chemical moieties enables the rational design of probes for the precise interrogation of biochemical and biological phenomena.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures





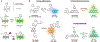

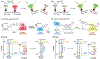





References
-
- de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT and Rice TE, Chemical Reviews, 1997, 97, 1515–1566. - PubMed
-
- Johnson I, The Histochemical Journal, 1998, 30, 123–140. - PubMed
-
- Staderini M, Martín MA, Bolognesi ML and Menéndez JC, Chemical Society Reviews, 2015, 44, 1807–1819. - PubMed
-
- Alamudi SH and Chang Y-T, Chemical Communications, 2018, 54, 13641–13653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

