SARS-CoV-2 S1 is superior to the RBD as a COVID-19 subunit vaccine antigen
- PMID: 32691875
- PMCID: PMC7404424
- DOI: 10.1002/jmv.26320
SARS-CoV-2 S1 is superior to the RBD as a COVID-19 subunit vaccine antigen
Abstract
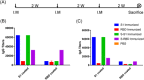
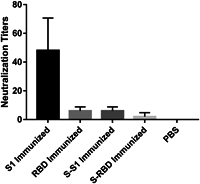
Since its emergence in December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has developed into a global pandemic within a matter of months. While subunit vaccines are one of the prominent options for combating coronavirus disease 2019 (COVID-19), the immunogenicity of spike protein-based antigens remains unknown. When immunized in mice, the S1 domain induced much higher IgG and IgA antibody levels than the receptor-binding domain (RBD) and more efficiently neutralized SARS-CoV-2 when adjuvanted with alum. It is inferred that a large proportion of these neutralization epitopes are located in the S1 domain but outside the RBD and that some of these are spatial epitopes. This finding indicates that expression systems with posttranslational modification abilities are important to maintain the natural configurations of recombinant spike protein antigens and are critical for effective COVID-19 vaccines. Further, adjuvants prone to a Th1 response should be considered for S1-based subunit COVID-19 vaccines to reduce the potential risk of antibody-dependent enhancement of infection.
Keywords: COVID-19; S1; SARS-CoV-2 subunit vaccine; antibody-dependent enhancement; receptor-binding domain; spike protein.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures

Similar articles
-
Adjuvants to the S1-subunit of the SARS-CoV-2 spike protein vaccine improve antibody and T cell responses and surrogate neutralization in mice.Sci Rep. 2024 Nov 28;14(1):29609. doi: 10.1038/s41598-024-80636-3. Sci Rep. 2024. PMID: 39609527 Free PMC article.
-
A two-dose optimum for recombinant S1 protein-based COVID-19 vaccination.Virology. 2022 Jan;566:56-59. doi: 10.1016/j.virol.2021.11.011. Epub 2021 Dec 1. Virology. 2022. PMID: 34864488 Free PMC article.
-
Adjuvanting a subunit COVID-19 vaccine to induce protective immunity.Nature. 2021 Jun;594(7862):253-258. doi: 10.1038/s41586-021-03530-2. Epub 2021 Apr 19. Nature. 2021. PMID: 33873199
-
Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects.Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. Rev Med Virol. 2021. PMID: 33594794 Free PMC article. Review.
-
SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants.Front Immunol. 2021 Jul 12;12:701501. doi: 10.3389/fimmu.2021.701501. eCollection 2021. Front Immunol. 2021. PMID: 34322129 Free PMC article. Review.
Cited by
-
Trichinella spiralis Infection Inhibits the Efficacy of RBD Protein of SARS-CoV-2 Vaccination via Regulating Humoral and Cellular Immunity.Vaccines (Basel). 2024 Jun 30;12(7):729. doi: 10.3390/vaccines12070729. Vaccines (Basel). 2024. PMID: 39066367 Free PMC article.
-
Involvement of the STING signaling in COVID-19.Front Immunol. 2022 Dec 8;13:1006395. doi: 10.3389/fimmu.2022.1006395. eCollection 2022. Front Immunol. 2022. PMID: 36569928 Free PMC article. Review.
-
Nanoparticular CpG-adjuvanted SARS-CoV-2 S1 protein elicits broadly neutralizing and Th1-biased immunoreactivity in mice.Int J Biol Macromol. 2021 Dec 15;193(Pt B):1885-1897. doi: 10.1016/j.ijbiomac.2021.11.020. Epub 2021 Nov 11. Int J Biol Macromol. 2021. PMID: 34774590 Free PMC article.
-
An Established Th2-Oriented Response to an Alum-Adjuvanted SARS-CoV-2 Subunit Vaccine Is Not Reversible by Sequential Immunization with Nucleic Acid-Adjuvanted Th1-Oriented Subunit Vaccines.Vaccines (Basel). 2021 Nov 1;9(11):1261. doi: 10.3390/vaccines9111261. Vaccines (Basel). 2021. PMID: 34835192 Free PMC article.
-
SARS-CoV-2 protein subunit vaccination of mice and rhesus macaques elicits potent and durable neutralizing antibody responses.Cell Rep Med. 2021 Apr 20;2(4):100252. doi: 10.1016/j.xcrm.2021.100252. Epub 2021 Apr 5. Cell Rep Med. 2021. PMID: 33842900 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

