Physiologically-Based Pharmacokinetic Modeling Characterizes the CYP3A-Mediated Drug-Drug Interaction Between Fluconazole and Sildenafil in Infants
- PMID: 32691891
- PMCID: PMC8138939
- DOI: 10.1002/cpt.1990
Physiologically-Based Pharmacokinetic Modeling Characterizes the CYP3A-Mediated Drug-Drug Interaction Between Fluconazole and Sildenafil in Infants
Abstract
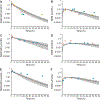
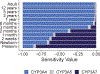
Physiologically-based pharmacokinetic (PBPK) modeling can potentially predict pediatric drug-drug interactions (DDIs) when clinical DDI data are limited. In infants for whom treatment of pulmonary hypertension and prevention or treatment of invasive candidiasis are indicated, sildenafil with fluconazole may be given concurrently. To account for developmental changes in cytochrome P450 (CYP) 3A, we determined and incorporated fluconazole inhibition constants (KI ) for CYP3A4, CYP3A5, and CYP3A7 into a PBPK model developed for sildenafil and its active metabolite, N-desmethylsildenafil. Pharmacokinetic (PK) data in preterm infants receiving sildenafil with and without fluconazole were used for model development and evaluation. The simulated PK parameters were comparable to observed values. Following fluconazole co-administration, differences in the fold change for simulated steady-state area under the plasma concentration vs. time curve from 0 to 24 hours (AUCss,0-24 ) were observed between virtual adults and infants (2.11-fold vs. 2.82-fold change). When given in combination with treatment doses of fluconazole (12 mg/kg i.v. daily), reducing the sildenafil dose by ~ 60% resulted in a geometric mean ratio of 1.01 for simulated AUCss,0-24 relative to virtual infants receiving sildenafil alone. This study highlights the feasibility of PBPK modeling to predict DDIs in infants and the need to include CYP3A7 parameters.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
CONFLICTS OF INTEREST
M.M.L. has received support for work on data safety and monitoring boards and drug development from Medipost, United Therapeutics, and Aguettant. All other authors declared no competing interests for this work.
Figures

References
-
- US Food and Drug Administration Center for Drug Evaluation and Research. Clinical drug interaction studies-cytochrome P450 enzyme- and transporter-mediated drug interactions. Guidance for industry <https://www.fda.gov/media/134581/download> (2020). Accessed August 5, 2020.
-
- Stevens JC et al. Developmental expression of the major human hepatic CYP3A enzymes. J. Pharmacol. Exp. Ther 307, 573–582 (2003). - PubMed
-
- Sim SC, Edwards RJ, Boobis AR & Ingelman-Sundberg M. CYP3A7 protein expression is high in a fraction of adult human livers and partially associated with the CYP3A7*1C allele. Pharmacogenet. Genomics 15, 625–631 (2005). - PubMed
-
- Williams JA et al. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab. Dispos 30, 883–891 (2002). - PubMed
-
- Gibbs MA, Thummel KE, Shen DD & Kunze KL Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: comparison of Ki values and impact of CYP3A5 expression. Drug Metab. Dispos 27, 180–187 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

