Hepatic 31 P-magnetic resonance spectroscopy identified the impact of melatonin-pretreated mitochondria in acute liver ischaemia-reperfusion injury
- PMID: 32691975
- PMCID: PMC7520314
- DOI: 10.1111/jcmm.15617
Hepatic 31 P-magnetic resonance spectroscopy identified the impact of melatonin-pretreated mitochondria in acute liver ischaemia-reperfusion injury
Abstract
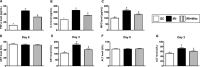
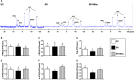
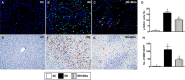
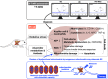
Acute liver ischaemia-reperfusion injury (IRI), commonly encountered during liver resection and transplantation surgery, is strongly associated with unfavourable clinical outcome. However, a prompt and accurate diagnosis and the treatment of this entity remain formidable challenges. This study tested the hypothesis that 31 P-magnetic resonance spectroscopy (31 P-MRS) findings could provide reliable living images to accurately identify the degree of acute liver IRI and melatonin-pretreated mitochondria was an innovative treatment for protecting the liver from IRI in rat. Adult male SD rats were categorized into group 1 (sham-operated control), group 2 (IRI only) and group 3 (IRI + melatonin [ie mitochondrial donor rat received intraperitoneal administration of melatonin] pretreated mitochondria [10 mg/per rat by portal vein]). By the end of study period at 72 hours, 31 P-MRS showed that, as compared with group 1, the hepatic levels of ATP and NADH were significantly lower in group 2 than in groups 1 and 3, and significantly lower in group 3 than in group 1. The liver protein expressions of mitochondrial-electron-transport-chain complexes and mitochondrial integrity exhibited an identical pattern to 31 P-MRS finding. The protein expressions of oxidative stress, inflammatory, cellular stress signalling and mitochondrial-damaged biomarkers displayed an opposite finding of 31 P-MRS, whereas the protein expressions of antioxidants were significantly progressively increased from groups 1 to 3. Microscopic findings showed that the fibrotic area/liver injury score and inflammatory and DNA-damaged biomarkers exhibited an identical pattern of cellular stress signalling. Melatonin-pretreated mitochondria effectively protected liver against IRI and 31 P-MRS was a reliable tool for measuring the mitochondrial/ATP consumption in living animals.
Keywords: 31P-magnetic resonance spectroscopy; liver ischaemia-reperfusion injury; melatonin; mitochondria.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures



References
-
- Busuttil R. Liver ischaemia and reperfusion injury. Br J Surg. 2007;94:787‐788. - PubMed
-
- Azbill RD, Mu X, Bruce‐Keller AJ, Mattson MP, Springer JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997;765:283‐290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

