The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury
- PMID: 32691994
- PMCID: PMC7695641
- DOI: 10.1002/sctm.19-0135
The leading edge: Emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury
Abstract
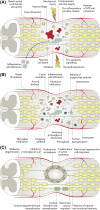
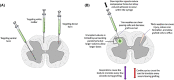
Spinal cord injuries (SCIs) are associated with tremendous physical, social, and financial costs for millions of individuals and families worldwide. Rapid delivery of specialized medical and surgical care has reduced mortality; however, long-term functional recovery remains limited. Cell-based therapies represent an exciting neuroprotective and neuroregenerative strategy for SCI. This article summarizes the most promising preclinical and clinical cell approaches to date including transplantation of mesenchymal stem cells, neural stem cells, oligodendrocyte progenitor cells, Schwann cells, and olfactory ensheathing cells, as well as strategies to activate endogenous multipotent cell pools. Throughout, we emphasize the fundamental biology of cell-based therapies, critical features in the pathophysiology of spinal cord injury, and the strengths and limitations of each approach. We also highlight salient completed and ongoing clinical trials worldwide and the bidirectional translation of their findings. We then provide an overview of key adjunct strategies such as trophic factor support to optimize graft survival and differentiation, engineered biomaterials to provide a support scaffold, electrical fields to stimulate migration, and novel approaches to degrade the glial scar. We also discuss important considerations when initiating a clinical trial for a cell therapy such as the logistics of clinical-grade cell line scale-up, cell storage and transportation, and the delivery of cells into humans. We conclude with an outlook on the future of cell-based treatments for SCI and opportunities for interdisciplinary collaboration in the field.
Keywords: clinical trials; neuroprotection; neuroregeneration; spinal cord injury; stem cells.
© 2020 The Authors. STEM CELLS TRANSLATIONAL MEDICINE published by Wiley Periodicals LLC on behalf of AlphaMed Press.
Conflict of interest statement
Michael G. Fehlings declared advisory role with Fortuna Fix. The other authors declared no potential conflicts of interest.
Figures

References
-
- Christopher & Dana Reeve Foundation . 2010. One degree of separation: paralysis and spinal cord injury in the United States.
-
- National Spinal Cord Injury Statistical Center . Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 2018.
-
- Ahuja CS, Nori S, Tetreault L, et al. Traumatic spinal cord injury‐repair and regeneration. Neurosurgery. 2017;80:S9‐S22. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

