Nuclear Membrane Rupture and Its Consequences
- PMID: 32692592
- PMCID: PMC8191142
- DOI: 10.1146/annurev-cellbio-020520-120627
Nuclear Membrane Rupture and Its Consequences
Abstract
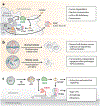
The nuclear envelope is often depicted as a static barrier that regulates access between the nucleus and the cytosol. However, recent research has identified many conditions in cultured cells and in vivo in which nuclear membrane ruptures cause the loss of nuclear compartmentalization. These conditions include some that are commonly associated with human disease, such as migration of cancer cells through small spaces and expression of nuclear lamin disease mutations in both cultured cells and tissues undergoing nuclear migration. Nuclear membrane ruptures are rapidly repaired in the nucleus but persist in nuclear compartments that form around missegregated chromosomes called micronuclei. This review summarizes what is known about the mechanisms of nuclear membrane rupture and repair in both the main nucleus and micronuclei, and highlights recent work connecting the loss of nuclear integrity to genome instability and innate immune signaling. These connections link nuclear membrane rupture to complex chromosome alterations, tumorigenesis, and laminopathy etiologies.
Keywords: ESCRT-III; TREX1; cGAS; chromothripsis; membrane dynamics; nuclear lamina.
Figures

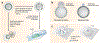

References
-
- Ablasser A, Chen ZJ. 2019. cGAS in action: expanding roles in immunity and inflammation. Science 363(6431):eaat8657. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

